Highlights
Mitochondria play a critical role in cellular function as the site of energy conversion. Dysfunction or dysregulation of mitochondria has been associated with aging-related and neurodegenerative diseases. These researchers studied mitochondrial stress signaling to better understand how mitochondria communicate stress to the rest of the cell. They successfully identified the major proteins involved in mitochondrial stress signaling including the proteins DELE1, OMA1, and HRI. In the future, these researchers hope to identify ways this mitochondrial signaling pathway can be altered. Their ultimate goal is to better understand the connections between mitochondrial stress and age-related and neurodegenerative disorders.
Did you know that over 90% of the oxygen we breathe is used by the mitochondria in our cells? Mitochondria are small
organelles
inside each cell where energy is converted and stored in adenosine triphosphate (ATP). Oxygen is a key component in this process, which is why an overwhelming amount of oxygen ends up in the mitochondria.
Although mitochondria are best known as the site of ATP production, they play many other roles that are important for cellular life. We know this because dysfunction or dysregulation of mitochondria is strongly associated with human disease. For example, there are over 350 genes whose mutation has been known to lead to mitochondrial disease. Most of these genes are encoded in the nuclear genome, but a small number of genes also reside in
mitochondrial DNA.
In March 2021, What A Year highlighted the work of Drs. Compton and Frazier at the Murdoch Children's Research Institute in Melbourne, Australia, who identified a novel mutation in mitochondrial DNA that causes heart failure and death in infants shortly before or after birth.
In addition to diseases caused by genetic mutations of mitochondrial or
nuclear DNA,
mitochondria have also been implicated in the aging process and sporadic age-related diseases such as
neurodegenerative diseases.
New research by Dr. Lucas Jae, Dr. Evelyn Fessler, Luisa Krumwiede, a master's student, and colleagues in the Jae Laboratory at Ludwig-Maximilians-University in Munich, Germany together with other collaborators offers new insight into how mitochondria are involved in age-related diseases. These researchers hope their work will support the development of novel therapies for such diseases in the future.
Mighty Mitochondria
Mitochondria are a unique type of organelle because their ancestors were bacteria that were engulfed by what would become
eukaryotic cells,
cells with a membrane-bound
nucleus
that are the building blocks of most plant and animal life. As a result of this evolutionary history, each mitochondrion has two membranes: the outer mitochondrial membrane and the inner mitochondrial membrane. Between the two membranes lies the intermembrane space, and within the inner mitochondrial membrane is the
mitochondrial matrix.
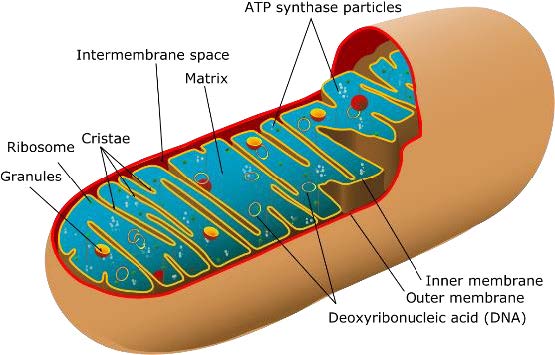
Figure 1. Anatomy of a mitochondrion.
[Source: https://commons.wikimedia.org/wiki/File:Animal_mitochondrion_diagram_en_%28edit%29.svg]
In addition, mitochondria have their own genetic information, a circular genetic strand that contains 37 genes in humans. However, despite the fact that mitochondria have their own DNA, most of their genetic information was transferred to the nucleus of the cell over the course of evolution.
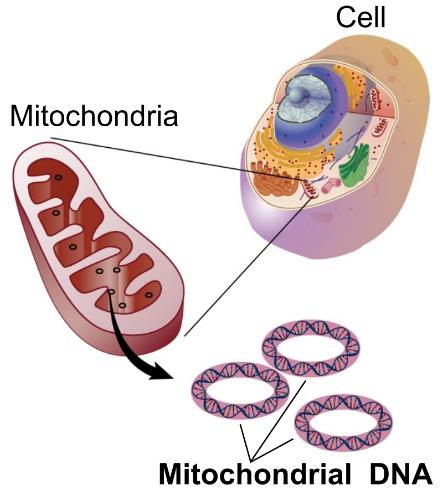
Figure 2. Circular mitochondrial DNA. Most of the genes regulating mitochondrial function are found in the nucleus of the cell.
[Source: https://en.wikipedia.org/wiki/Mitochondrial_DNA]
This means that most proteins related to the mitochondria are produced outside of the mitochondria itself and must then be transported through the cell to the mitochondria. This includes proteins that support the mitochondria while they are undergoing stress.
Mammalian cells have a mechanism to handle stress known as the
integrated stress response
or ISR. The ISR can be activated by stress in different parts of the cell. When the ISR is activated, certain cell functions change so the cell can respond to stress.
Since the genes that are able to activate the ISR and counteract mitochondrial stress are located in the nucleus and not the mitochondria, mitochondria must send signals to the cell nucleus via the
cytosol,
the liquid substance inside cells. This process, known as mito-nuclear communication, has been well studied in simpler organisms like yeast and worms. However, the pathways in humans are much less understood.
CHOP and the Integrated Stress Response
To better understand how mitochondria activate the ISR, Drs. Fessler and Jae used a genome wide screening strategy. This strategy allows the researchers to identify genes that are either positively or negatively associated with the activity and fate of a specific protein. To start, the researchers focused on the protein CHOP, which was a protein known to be involved in the ISR.
Using the genome wide screening strategy, the researchers created a cellular library where each cell was missing a gene. They then measured the level of CHOP expression for each cell entry in the library to determine which cells altered the CHOP protein. Failure to induce CHOP expression in response to mitochondrial stress would indicate a positive connection between the gene that was missing in the cell entry and CHOP. The results of this experiment identified a suite of proteins with a positive relationship to CHOP, including OMA1, DELE1, and HRI.
The researchers knew that OMA1 was a protease, an enzyme that functions to cleave proteins, located in the mitochondrial inner membrane and HRI was an ISR-related enzyme located in the cytosol. DELE1 had also been reported to localize to some part of the mitochondria, but otherwise little was known about DELE1.
Locating DELE1 Within the Mitochondria
The researchers conducted experiments to better understand where DELE1 was found within the mitochondria. Recall that there are four different locations for any protein within the mitochondria: the outer mitochondrial membrane, intermembrane space, inner mitochondrial membrane, and mitochondrial matrix. In order to enter the mitochondrial matrix, most mitochondrial proteins have a special sequence that must be
cleaved
by a protein called the mitochondrial processing peptidase (MPP).
The researchers observed two types of the DELE1 protein: L-DELE1 and M-DELE1. If MPP was not present, the researchers found that M-DELE1 could no longer be detected, indicating that M-DELE1 is generated by MPP-mediated cleavage of L-DELE1. Thus, Dr. Fessler and colleagues hypothesized that DELE1 went to the mitochondrial matrix, but they needed experimental evidence.
To provide this evidence, the researchers chose a split fluorescence approach, where one part of a fluorescent protein was attached to DELE1, and the second part of the fluorescent protein was attached to a different protein known to exist in the mitochondrial matrix. Both proteins needed to be in the same place in order for the fluorescent signal to occur. The researchers observed fluorescence when DELE1 was co-expressed with other matrix proteins, indicating the matrix as a destination for DELE1.
To corroborate the location of DELE1 within mitochondria, the researchers used a technique called proteinase K protection assay. Proteinase K is an enzyme that can degrade almost any kind of protein. The researchers exposed mitochondria isolated from human cells to proteinase K in three conditions:
- Intact mitochondrion. When the mitochondria in this condition were exposed to proteinase K, only proteins that reside in the outer mitochondrial membrane were degraded by the enzyme.
- Partially open mitochondrion. When the mitochondria in this condition were exposed to proteinase K, proteins in the outer mitochondrial membrane, intermembrane space, and inner mitochondrial membrane were degraded by the enzyme.
- Completely open mitochondrion. When the mitochondria in this condition were exposed to proteinase K, proteins in all areas of the mitochondria including the mitochondrial matrix were degraded by the enzyme.
By measuring the amounts of protein degraded versus intact, these experiments provided further evidence that DELE1 was predominantly found in the mitochondrial matrix. Surprisingly, the researchers observed that a proportion of DELE1 was also found protruding from the outer mitochondrial membrane. The researchers hypothesized that they were observing the process of DELE1 entering the mitochondrion, which is why DELE1 was found in both places.
DELE1 and Mitochondrial Stress
Next, the researchers wanted to understand how the DELE1 protein, which gets imported deep into mitochondria in healthy cells, can lead to activation of the ISR via HRI, which is located in the cytosol. Their experiments showed that upon mitochondrial stress, the DELE1 protein is processed by OMA1 into a shorter fragment termed S-DELE1. In addition to this cleavage event, DELE1 relocates when mitochondria are stressed and can be found in the cytosol.
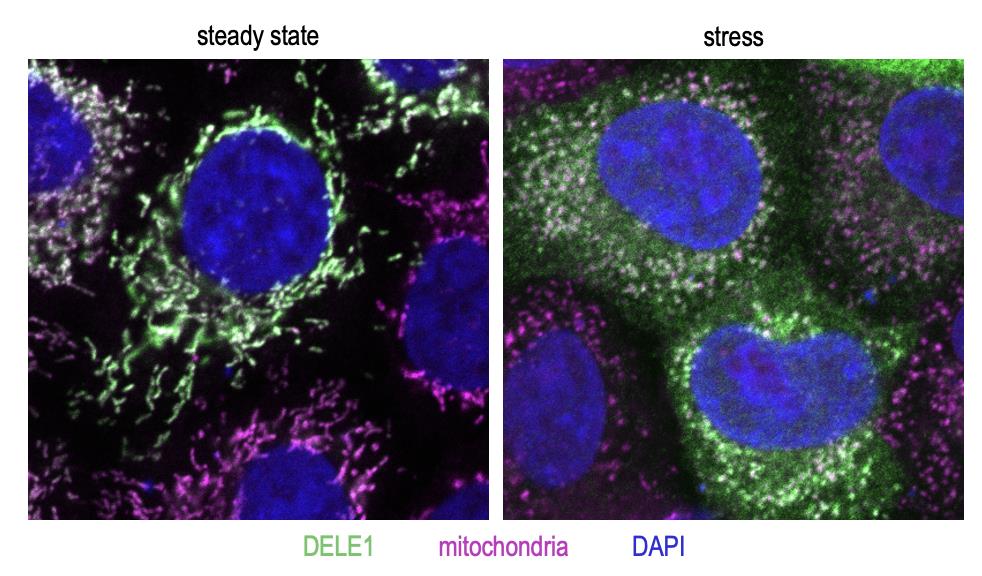
Figure 3. The DELE1 protein (green) localizes to mitochondria (magenta) in unstressed cells (left). Upon stress (in this case, dissipation of mitochondrial membrane potential using the chemical carbonyl cyanide m-chlorophenyl hydrazone or CCCP), DELE1 translocates to the cytosol (right).
[Source: Dr. Fessler]
To understand how OMA1 can gain access to DELE1 and how DELE1 can translocate to the cytosol, the researchers conducted the proteinase K experiment, this time with mitochondria that had experienced stress. One way to cause mitochondrial stress is by using the chemical carbonyl cyanide m-chlorophenyl hydrazone (CCCP), which blocks import across the inner mitochondrial membrane by removing the differences in electrical charge. Under this type of stress, M-DELE1 was not observed at all because the L-DELE1 protein could no longer reach the mitochondrial matrix. L-DELE1 was found to be sensitive to proteinase K in all three conditions, meaning in intact, partially opened, and completely opened mitochondria.
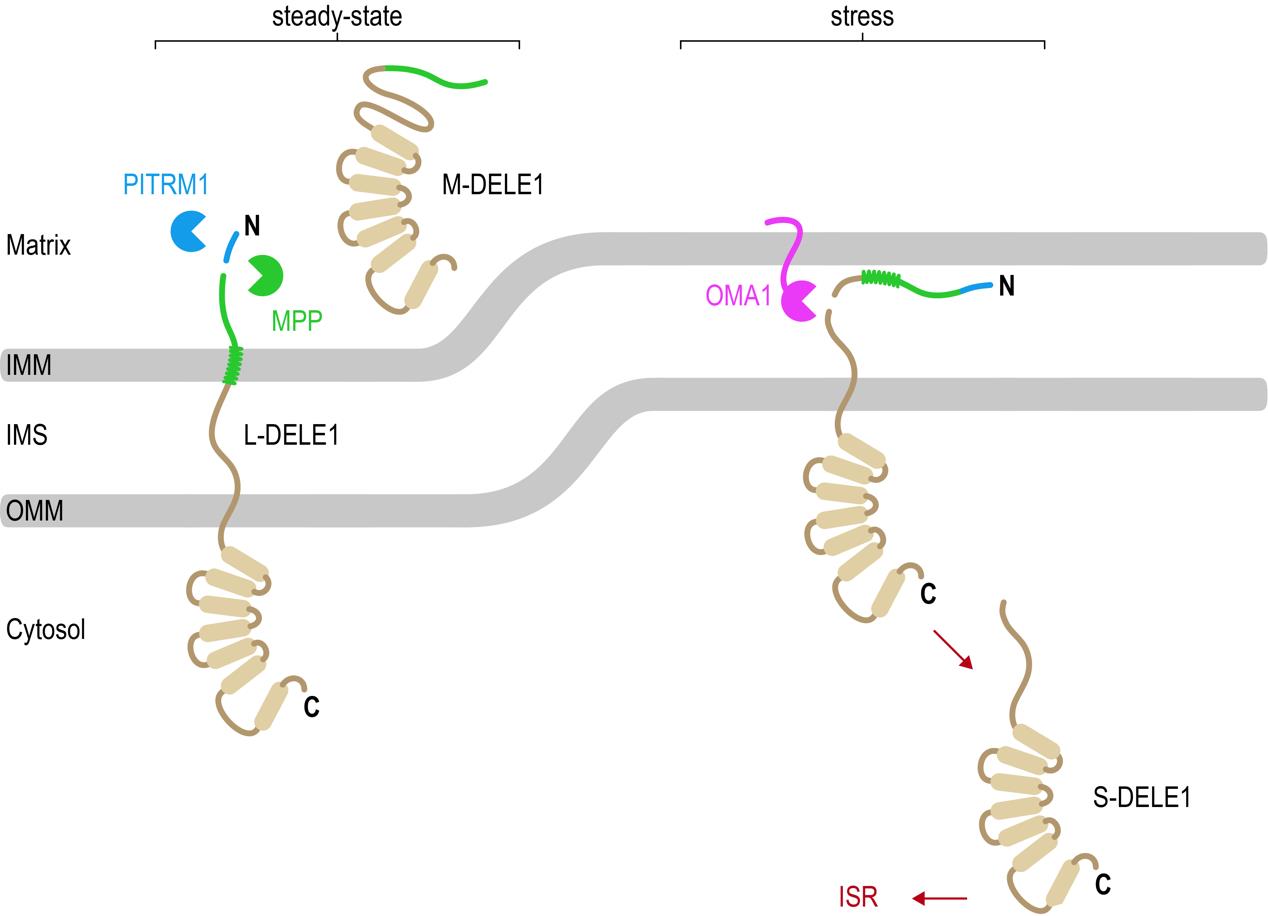
Figure 4. Newly synthesized DELE1 molecules are continuously imported across both mitochondrial membranes into the mitochondrial matrix. The mitochondrial processing peptidase (MPP) cleaves the precursor L-DELE1 protein, generating the mature M-DELE1 species. Presequences removed by MPP are degraded by PITRM1. Upon stress (for example dissipation of mitochondrial membrane potential using CCCP), the DELE1 protein is arrested during import, allowing the OMA1 protease to access the DELE1 protein and to cleave off its N-terminus. This generates the short version S-DELE1, which translocates to the cytosol and binds HRI to activate the ISR.
[Source: Dr. Fessler]
“These results explain how DELE1 can move from mitochondria to the cytosol and how DELE1 and OMA1 interact with each other,” explained Dr. Fessler. “Mitochondrial stress leads to defects in the import of DELE1 such that DELE1 becomes arrested across the mitochondrial membrane(s) with part of it remaining in the cytosol. This way the DELE1 protein becomes available for OMA1 to cleave into S-DELE1. S-DELE1 can then slip back into the cytosol where it binds to HRI leading to its activation.”
Mitochondrial Stress Signaling and Human Disease
Dr. Fessler and Luisa hypothesized that defects in mitochondrial stress signaling pathways may be one cause for mitochondria-related aging and neurodegenerative diseases. Two mitochondrial proteins known to be associated with neurodegenerative disorders are mitochondrial processing peptidase (MPP) noted above and pitrilysin metallopeptidase 1 (PITRM1). In patients, PITRM1 mutations have been shown to lead to less protein activity.
To investigate the role of DELE1 signaling in the absence of PITRM1, the researchers used a competitive growth assay. Luisa compared two cell lines: one without PITRM1, and one without both PITRM1 and DELE1. The two cell lines also had fluorescent markers so the researchers could tell them apart. Watching cell growth, Luisa observed the cells that still had DELE1 significantly outgrew those that did not have DELE1. This shows the importance of DELE1 signaling to cellular survival in this setting.
“We now know the key players in this pathway and how they work together to signal mitochondrial stress,” remarked Dr. Fessler. “Now we want to know how we might alter the pathway to improve cellular function.” In future experiments Dr. Fessler hopes to better understand the mitochondrial stress signaling pathway and learn under what other conditions stress signaling can be harmful to cells, and when it can be beneficial.
“The hope is that we will one day be able to modulate the pathway so it is beneficial for human health and can restore function,” explained Dr. Fessler. “Our ultimate goal is to improve our understanding of mitochondria-related aging and neurodegenerative disorders to inspire new therapeutic strategies.”
Dr. Evelyn Fessler is a postdoctoral researcher in the laboratory of Dr. Lucas Jae at Ludwig-Maximilians-University in Munich, Germany. Her research focuses on the genetic basis of mitochondrial stress underlying aging and neurodegenerative diseases. When not in the laboratory, Dr. Fessler enjoys hiking and reading.
Luisa Krumwiede is a master's student working with Dr. Fessler in the laboratory of Dr. Lucas Jae. When not in the laboratory, Luisa enjoys spending time outside hiking, biking, and swimming.
Dr. Lucas Jae is Professor of Functional Genomics at Ludwig-Maximilians-University in Munich, Germany. His research focuses on how genes shape cellular function using a wide array of genetic techniques. When not in the laboratory, Dr. Jae enjoys music and nature.
For More Information:
- Fessler, E., L. Krumwiede, and L. Jae. 2022. “DELE1 tracks perturbed protein import and processing in human mitochondria.” 13:1853. https://www.nature.com/articles/s41467-022-29479-y
- Eckl, E.-M. et al. 2021. “Sensing, signaling, and surviving mitochondrial stress.” 78: 5925-51. https://link.springer.com/article/10.1007/s00018-021-03887-7
- Fessler, E. et al. 2020. “A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol.” 579: 433-7. https://www.nature.com/articles/s41586-020-2076-4
To Learn More:
- Jae Laboratory. https://www.genzentrum.uni-muenchen.de/research-groups/jae/index.html
- Primary Mitochondrial disorders. https://www.ncbi.nlm.nih.gov/books/NBK1224/
- The Origin of Mitochondria. https://www.nature.com/scitable/topicpage/the-origin-of-mitochondria-14232356/
- What is DNA. https://medlineplus.gov/genetics/understanding/basics/dna/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

