Highlights
Sugar substitutes, also called non-nutritive sweeteners (NNS) are popular sugar replacements to get a sweet taste without the calories. The U.S. Food and Drug Administration has approved eight NNS for consumption in the United States. However, the potential health effects of NNS consumption during pregnancy and in people taking certain medications remain unknown. These researchers found that in mice, NNS consumption during pregnancy led to liver toxicity in offspring. They then conducted experiments in cell-free environments and liver cells to better understand how consumption of NNS affects liver function. They found that NNS consumption can disrupt liver function by preventing the protein P-glycoprotein from removing toxins from the liver. In future work the researchers plan to expand their study to include additional sweeteners as well as further investigate the specific dosages of NNS at which liver function is impacted. Further research is needed to translate these findings into guidance for clinicians and patients.
Did you know that the average child in the United States consumes over 65 pounds of sugar each year? That's enough sugar to fill a bathtub! While our bodies do need sugar in small quantities, excessive sugar consumption can lead to health problems such as
obesity
and
diabetes.
For this reason, many people have turned to artificial sweeteners, also called
non-nutritive sweeteners (NNS)
that taste like sugar but don't add calories to the diet.
The U.S. Food and Drug Administration (FDA) has approved eight NNS including aspartame, acesulfame potassium, sucralose, and stevia.

Figure 1. Common artificial sweeteners. The brand Equal is made from aspartame; Sweet and Low is made from saccharin, and Splenda is made from sucralose.
[Source: https://en.wikipedia.org/wiki/Sugar_substitute]
If you look at the ingredients in common foods and beverages, you will likely see some of these sweeteners listed. However, the health effects of NNS consumption remain unknown. New research by Dr. Stephanie Olivier-Van Stichelen, Assistant Professor of Biochemistry at Medical College of Wisconsin, and doctoral candidate Laura Danner shows that NNS consumption may negatively impact liver function. This is an important step in understanding how NNS may impact our health, which the researchers hope will enable clinicians and patients to make more informed decisions about the risks and benefits of NNS consumption.
Liver Detoxification and P-glycoprotein
The liver is an essential organ in the body that is involved in digestion, energy storage, and maintaining the balance of nutrients in the bloodstream. The liver is also responsible for breaking down toxins so they can safely be removed from the body.
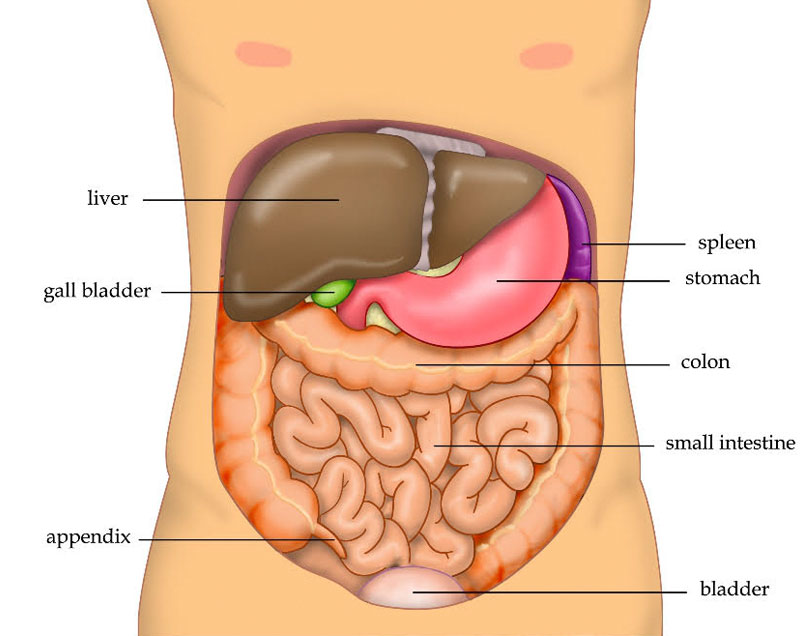
Figure 2. The liver sits above the gall bladder and stomach in the upper abdomen.
[Source: https://en.wikipedia.org/wiki/Liver]
Toxins can be compounds produced within the body, byproducts of compounds that have been consumed or produced by the body, and compounds that have been consumed and not metabolized. Compounds are said to be toxic when they are more reactive, which could lead to cell damage if they are not removed.
The process by which the liver detoxifies compounds can be broken down into roughly three phases:
- Phase I - The liver receives blood from the bloodstream and converts toxins into less reactive forms
- Phase II - Molecules are added to the less reactive forms to make them easier to excrete from the body via urine and stool
- Phase III - The altered compounds are removed from the liver cells for excretion
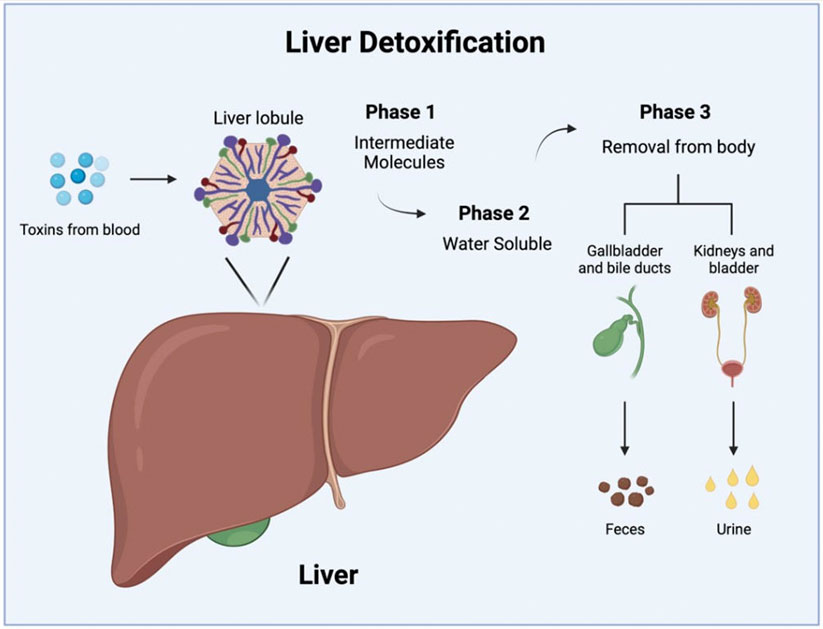
Figure 3. Liver detoxification process.
[Source: Dr Olivier-Van Stichelen. Created with BioRender.com]
One protein known to be important to liver detoxification is called P-glycoprotein or (PGP). PGP is also known as Multidrug Resistance Protein 1 (MDR1) due to its harmful function when found in cancerous tumors. In healthy organs, PGP is found in many parts of the body where its role is to remove toxins from the cell. For example, PGP transports certain medicines and toxic compounds absorbed from food back to the intestines for removal from the body. PGP is also found in the
placenta
where it protects the growing fetus from toxins while the fetal liver is still maturing.
In the liver, PGP is involved primarily in Phase III of detoxification. It sits on the membrane of liver cells and looks for toxic compounds that need to be removed. PGP removes toxic compounds by binding to them and transporting them across the cellular membrane to be excreted in
bile.
If PGP is not working properly, toxins may remain inside the cell for prolonged periods of time, where they could cause damage to the functioning of other cells and tissues.
NNS Consumption and the Liver
Everything we consume that does not get metabolized in the body will at some point be removed via the liver, including certain medications. Medications such as antidepressants, antibiotics, and blood pressure medications use PGP as the primary transporter for detoxification and removal from the body. For this reason, Dr. Olivier-Van Stichelen and Laura were interested in how NNS consumption might affect liver function, and PGP function specifically.
In particular, Dr. Olivier-Van Stichelen and Laura focused on two of the eight NNS approved by the FDA: acesulfame potassium and sucralose. They did this for two main reasons. First, acesulfame potassium and sucralose are two of the most commonly used NNS in food and beverages today. Second, acesulfame potassium and sucralose are not metabolized by the body. These sweeteners are found in high concentrations in the digestive tract and absorbed into the bloodstream after eating, where they come in contact with the liver. In contrast, other sweeteners, such as aspartame, undergo extensive processing and degradation in the digestive tract before getting to the liver, so they are less likely to impact liver function.
It was important to the researchers to test the two NNS individually and also in combination. This is because the initial safety testing conducted by the FDA on these compounds only looked at their safety individually but not together. However, acesulfame potassium and sucralose are often used together in products. Acesulfame potassium is commonly used because it is cheap to produce, but it can leave a bad aftertaste. For this reason, acesulfame potassium is typically used with sucralose, which helps to smooth out the flavor and remove the aftertaste.
Previous experiments by students in Dr. Olivier-Van Stichelen's laboratory had shown that NNS impacted the expression of the gene that encodes for PGP, indicating that NNS might disrupt normal PGP function. Laura continued these experiments by looking specifically at the impact of NNS on PGP activity.
First, the researchers exposed liver cells to different concentrations of acesulfame potassium and sucralose, individually and together. They used fluorescent signals as a marker of PGP function. The results showed that both sweeteners prevented PGP from functioning properly.
Based on these results, Dr. Olivier-Van Stichelen and Laura hypothesized that NNS interferes with PGP function because it binds to PGP and takes the place of a toxic compound, such as a medication. If PGP is not available for detoxification because it has already bound to NNS, this could prevent PGP from functioning to remove true toxins.
Having shown that NNS does impact normal PGP function in liver cells, the researchers next conducted experiments in a cell-free environment. Purified PGP proteins were embedded into artificial membranes. By observing changes in light signals (luminescence), the researchers measured how exposing PGP to NNS might change its function. They found that both individually and combined with acesulfame potassium, sucralose stimulated PGP function in the way a toxin or medication would. That means it could compete with toxins for removal from cells.
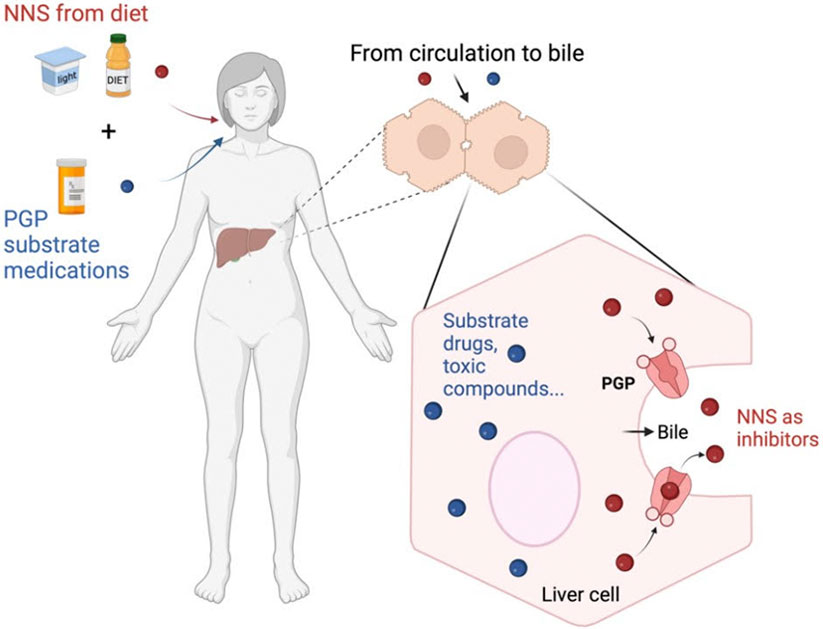
Figure 4. How NNS impact PGP function in the liver. NNS bind to PGP rather than other toxic compounds such as medications. Substrate refers to any compound that binds to PGP.
[Source: Dr. Olivier-Van Stichelen. Created with BioRender.com]
The effects of the two sweeteners were not the same. In general, sucralose had a stronger impact on PGP function than acesulfame potassium. “This is not surprising because the two sweeteners are very different from a chemical perspective,” Laura explained. Sucralose is a large molecule that looks very similar to the types of toxins that PGP usually binds. In contrast, acesulfame potassium is a smaller molecule that doesn't bind as well to PGP. Its process of preventing PGP function might be different from sucralose.
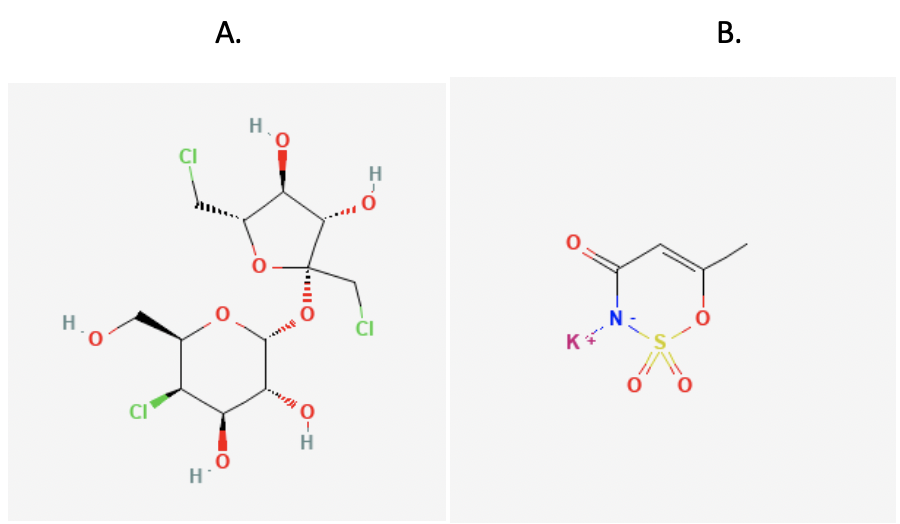
Figure 5. Chemical structures of A. sucralose and B. acesulfame potassium. Sucralose is a much larger molecule than acesulfame potassium.
[Sources: https://pubchem.ncbi.nlm.nih.gov/compound/Sucralose#section=2D-Structure; https://pubchem.ncbi.nlm.nih.gov/compound/Acesulfame-potassium#section=2D-Structure]
Future Work
The next step for this research is to more precisely quantify the specific dosage of NNS that causes the observed effects on PGP. The current FDA-recommended limits for NNS consumption are quite high. The highest recommended daily consumption of acesulfame potassium is equivalent to about 35 diet sodas and sucralose is equivalent to about 8 diet sodas. However, Dr. Olivier-Van Stichelen and Laura have found that significant effects on PGP function can be observed at much lower levels, such as the equivalent of one diet soda.
Another area of future work focuses on PGP in the placenta, which serves to protect the growing fetus from potential toxins while the liver is still maturing. Previous research has shown that the lack of PGP function in the placenta can lead to an increase in
birth defects.
Dr. Olivier-Van Stichelen and Laura hope to better understand how NNS consumption might impact the health of both mother and baby.
Finally, Dr. Olivier-Van Stichelen and Laura also want to expand their research on NNS to include stevia. Like sucralose, stevia is a large compound that resembles the types of compounds PGP usually binds. However, they realize it will be more complicated to measure the effects of stevia on the liver because it is partially metabolized by the body.
Laura Danner is a Ph.D. student in the laboratory of Dr. Olivier-Van Stichelen. Her research interests include how diet and changes in diet can impact health and disease. Her current research focuses on NNS consumption and how it might affect liver function. When not in the laboratory, Laura enjoys reading, being outdoors, and spending time with her dog and cat.
Dr. Stephanie Olivier-Van Stichelen is Assistant Professor of Biochemistry at Wisconsin Medical College. Her research focuses on how sugar and artificial sweeteners impact health and disease at different stages of development. When not in the laboratory, Dr. Olivier-Van Stichelen enjoys being active outdoors, camping, and spending time with her family.
To Learn More:
PGP and NNS:
- “Researchers uncover how non-nutritive sweeteners disrupt liver detoxification.” Medical College of Wisconsin. https://www.mcw.edu/newsroom/news-articles/researchers-uncover-how-nonnutritive-sweeteners-disrupt-liver-detoxification
- Danner L, Valdes R, Olivier-Van Stichelen S. 2022. “Non-nutritive sweeteners competitively inhibit P-glycoprotein in liver.“ FASEB J. 36 Suppl 1. https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.2022.36.S1.R4915
- Olivier-Van Stichelen, S., Rother, K. and Hanover, J. 2019. “Maternal Exposure to Non-nutritive Sweeteners Impacts Progeny's Metabolism and Microbiome.“Frontiers in Microbiology. https://pubmed.ncbi.nlm.nih.gov/31281295/
PGP and Pregnancy:
- Ellfolk, M. et al. 2020. “Placental transporter-mediated drug interactions and offspring congenital anomalies.“ British Journal of Clinical Pharmacology, 86(5): 868-79. https://pubmed.ncbi.nlm.nih.gov/31823387/
- Wang, Ch. Et al. 2013. “Increased risk for congenital heart defects in children carrying the ABCB1 Gene C3435T polymorphism and maternal periconceptional toxicants exposure.“ PLoS One, 8(7):e68807. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0068807
- Mölsä, M. et al. 2005. “Functional role of P-glycoprotein in the human blood-placental barrier.“ Clinical Pharmacology and Therapeutics, 78(2):123-31. https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1016/j.clpt.2005.04.014
- Smit, J. et al. 1999. “Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure.“ Journal of Clinical Investigation, 104(10):1441-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC409845/
- Lankas, G. et al. 1998. “Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice.“ Reproductive Toxicology, 12(4):457-63. https://www.sciencedirect.com/science/article/abs/pii/S0890623898000276?via%3Dihub
For More Information:
- The Olivier-Van Stichelen Lab @ Medical College of Wisconsin. https://www.ovsoglcnaclab.com/
- Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/liver-anatomy-and-functions#:~:text=All%20the%20blood%20leaving%20the,body%20or%20that%20are%20nontoxic.
- U.S. Food and Drug Administration. https://www.fda.gov/consumers/consumer-updates/how-sweet-it-all-about-sugar-substitutes
- Canadian Liver Foundation. https://www.liver.ca/your-liver/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

