| |
Highlights
These researchers used fluorescent probes to identify bacteria without a cell wall (called L-form) in the urine of patients with recurring urinary tract infections. Most urinary tract infections are treated with a full course of antibiotics. These researchers discovered that L-form bacteria can avoid detection by antibiotics that target the cell wall and revert to a normal walled form after the antibiotics are finished, causing a recurring infection. These findings open new pathways for approaching antibiotic resistance and the treatment of recurring infectious diseases.
Did you know that the human body contains about 10,000 distinct species of bacteria? Outside of our bodies, there are millions of species of bacteria, and most of them have not been identified by humans. Bacteria are everywhere, and yet we know so little about them, even the few species that cause major human illnesses. It is important for us to know more about bacteria, for the health and safety of our species and the world.
One characteristic that most bacterial species have in common is the presence of a
cell wall.
The bacterial cell wall is made of
peptidoglycan,
a mesh layer of sugars and
amino acids
linked together. The cell wall surrounds the bacterial cell, providing shape and structure, and facilitating replication.
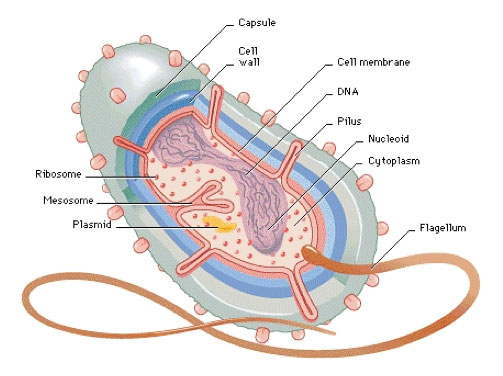
Figure 1. Anatomy of a generic bacterium
[Source: http://nsrscience.blogspot.com/2005/03/anatomy-of-simple-bacterium-bacteria.html
]
Human cells are surrounded directly by a membrane and do not have a cell wall. As a result, walled bacteria can easily be recognized as “foreign” by our immune system. In addition, the cell wall is a great target for many antibiotics, including penicillin, because such antibiotics specifically target the cell wall of bacteria, and do not cause harm to humans.
Despite its importance, bacteria can survive without the cell wall. Emmy Klieneberger was the first scientist to identify bacteria without a cell wall under the microscope in 1935. She named these bacterial forms L-form after the institution she worked for, the Lister Institute of London. Since then, scientists have been studying the formation of L-form bacteria and the transitions between L-form bacteria and walled bacteria.
Figure 2. Growing bacteria on agar plates
[Source: Dr. Katarzyna Mickiewicz]
Many scientists have hypothesized that L-form switching might help bacteria survive treatment with antibiotics that target the cell wall and contribute to recurrent infection, but evidence for this was hard to obtain. L-form bacteria are very challenging to isolate from humans for several reasons. First, L-form bacteria are delicate and susceptible to bursting in the absence of the cell wall. Second, without the cell wall, L-form bacteria look just like pieces of dead human cells. This made it difficult for researchers to tell what exactly they were seeing under the microscope. Finally, the number of L-form bacteria in human samples might be low and hard to find, as L-forms do not reproduce as efficiently as walled bacteria.
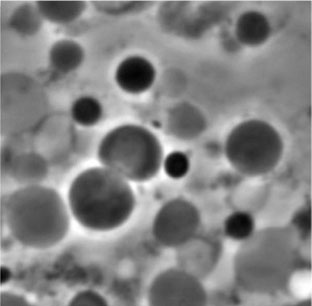
Figure 3. L-form bacteria
[Source: Dr. Katarzyna Mickiewicz]
Now, over 80 years since L-form bacteria were first named, their presence in humans has been unambiguously confirmed. The research team led by Dr. Katarzyna Mickiewicz in the laboratory of Professor Jeff Errington at Newcastle University in England found L-form bacteria in the urine of patients with recurring
urinary tract infections.
These findings open new pathways for approaching
antibiotic resistance
and the treatment of recurring
infectious diseases.
Dr. Katarzyna Mickiewicz first heard about L-form bacteria while completing her Ph.D in
immunology
at Newcastle University. She thought her background in immunology might allow her to identify L-forms in humans. Up until this point, the presence of L-forms in humans was hypothesized but had never been proven. Dr. Mickiewicz approached Dr. Jeff Errington at Newcastle, who studies the basic biology of L-form bacteria, and asked him if he would support her doing this project. As Dr. Mickiewicz recalled, “he told me he had been looking for someone brave enough to do this project, and that I could go ahead and try.” Working closely with other members of the Errington lab, including L-form expert Dr. Yoshikazu Kawai, she spent the next six years doing just that.
Using the latest technology, Dr. Mickiewicz thought she could separate the L-form bacteria from the rest of the cellular debris. As she says, “we revisited old questions with new technology.” In her pursuit of L-form bacteria in humans, Dr. Mickiewicz was very lucky to find an ongoing study at Newcastle, led by Dr. Phillip Aldridge and Dr. Judith Hall, looking at recurring urinary tract infections. The
urinary tract
is a series of organs and tubes that connect the kidneys to the bladder,
ureters,
and
urethra
to release urine.
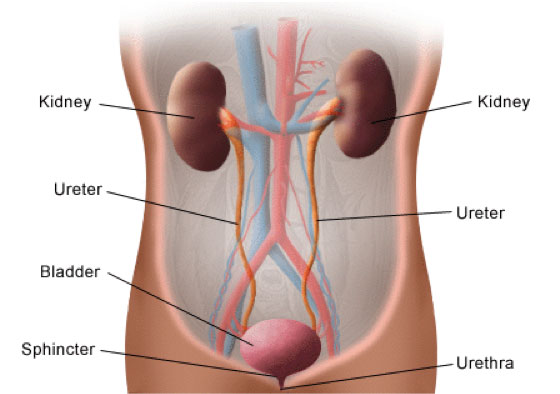
Figure 4. Anatomy of the urinary tract
[Source: https://www.hopkinsmedicine.org/health/wellness-and-prevention/anatomy-of-the-urinary-system]
Urinary tract infections (UTIs) occur when bacteria get into any part of the urinary tract and grow. Symptoms of UTIs include the frequent urge to urinate, pain or burning with urination, and strong-smelling or cloudy urine. UTIs are generally treated with
antibiotics.
Antibiotics are medicines used to treat bacterial infections, and different classes of antibiotics use different mechanisms to target and kill bacteria. For example, some antibiotics target the cell wall of the bacteria, while others target bacterial genetic information such as DNA. The most commonly prescribed antibiotic for UTIs are those targeting bacterial genetic information.
Most UTIs can be treated successfully with a full course of antibiotics. In some patients, though, the UTIs come back repeatedly, a condition known as recurring urinary tract infection or rUTI. One reason for the recurrence of UTIs is antibiotic resistance. This happens when the bacteria learn how to overcome or avoid the antibiotics.
One way to address this problem in patients with recurrent UTIs is to use a different type of antibiotic with a different mechanism of action. For example, if the first UTI was treated with an antibiotic targeting bacterial genetic material, the second UTI could be treated with a different antibiotic that targets the bacterial cell wall. In some patients, though, despite using a variety of antibiotics, the infection still comes back. In order to better understand the recurrence of UTIs, Drs. Aldridge and Hall developed a study to track patients with rUTIs over time.
In the study, 30 patients over 65 years of age provided urine samples every 2 weeks over 6 months. “This was a unique opportunity,” Dr. Mickiewicz recalls. “The researchers were not looking at L-forms, but they created the perfect experiment for us to do so.” The samples that Dr. Mickiewicz received were coded, so at the time they arrived into her laboratory, the researchers did not know any information about the samples they were testing, such as which patient the sample came from or whether the sample had tested positive for UTI in the clinical laboratory. In addition, the researchers received the sample right away, so there was no chance for the L-forms to degrade or disappear.
Once the samples reached Dr. Mickiewicz’s laboratory, the researchers used a technique called
fluorescence in situ hybridization (FISH)
to visualize the bacterial cells under the microscope. Bacterial cells were made visible by adding fluorescent markers that only attached to bacterial cells in fixed urine samples. This allowed the researchers to look at the bacterial cells using a high-powered fluorescent microscope (Figure 5). Using these markers, Dr. Mickiewicz was able to confirm that L-form bacteria were present in the urine samples of all but one patient in the study. “This was a huge breakthrough,” notes Dr. Mickiewicz. “No one had ever done this before.”
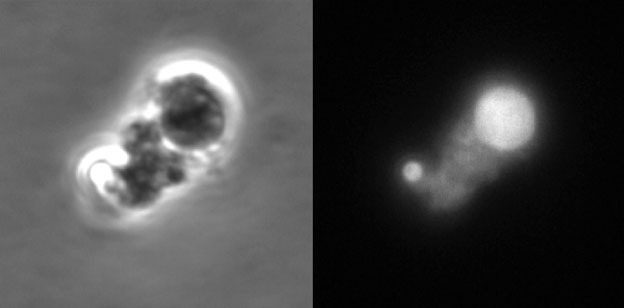
Figure 5. L-form bacteria identified in urine using a fluorescent probe. The left side shows L-form-like objects observed in patients’ urine using phase-contrast microscopy. The right side shows the same image visualized using fluorescence microscopy. The fluorescent signal was generated by a probe, which binds specifically to bacteria, and confirmed the bacterial origin of the structures visualized on the left side.
[Source: Dr. Katarzyna Mickiewicz]
Next, the researchers isolated the L-forms from other cellular debris in the sample using a fine mesh filter. Bacteria with cell walls are too big and rigid to go through the tiny holes of the filter, but the smaller, more flexible L-form bacteria do. Dr. Mickiewicz and her team filtered L-form bacteria into tubes containing
growth media,
which prevented the cells from bursting. They allowed the cells to grow for up to one month, which allowed the bacteria to regenerate the cell wall and quickly reproduce. Once the bacteria grew, the researchers could identify the particular species of bacteria. The majority of bacteria were E. coli, but the researchers also identified other species that commonly cause UTIs.
Antibiotic Evasion
Over the course of the study, Dr. Mickiewicz became very interested in the samples from a patient who received a type of antibiotic that targets the cell wall. After the experiment had been completed, the researchers found out that, when the samples from this patient were sent to the clinical laboratory to test for a UTI, the results came back negative. Despite the negative result, the researchers did find bacteria in the urine sample from this patient, only they were in the L-form without the cell wall. Dr. Mickiewicz believes that, at least in this case, the bacteria were avoiding antibiotics (and the laboratory test) by changing into the L-form, and that once the course of antibiotics was finished, the bacteria would revert to its normal walled form, causing a recurring infection.
Given this hypothesis, Dr. Mickiewicz wanted to see if she could cause the bacteria isolated from this patient to become L-form and then revert back to the normal walled cell under laboratory conditions. First, Dr. Mickiewicz took walled bacteria causing the UTI and placed them on a microscope slide in a medium that prevents any potential L-forms from bursting. Once the bacteria were growing, the researchers treated the cells with antibiotics and observed them in real time under the microscope. “In front of our eyes, the cells lost the cell wall and became L-form,” recalls Dr. Mickiewicz. At first, the bacteria divided normally, a process called
binary fission,
where one cell splits into two identical cells. Very soon, though, the cells began to lose their cell wall and became all sorts of shapes and sizes. When the antibiotic was removed, the same process happened in reverse. At first the L-form cells divided in their haphazard way, but very soon they began to regain the cell wall and divide according to binary fission.
Once Dr. Mickiewicz had shown that the bacteria could convert to L-form and then revert back in the artificial media, she wanted to see if this could happen in urine as well. The process was slower in urine, but still possible. Next, Dr. Mickiewicz did similar tests in living organisms.
The researchers used zebrafish larvae because they are transparent and can easily fit under the microscope. Dr. Mickiewicz injected walled bacterial cells into living zebrafish larvae under the microscope in the presence of antibiotics. Just as they had in the artificial conditions and in urine, the bacterial cells in the zebrafish lost their cell walls and became L-form. When the antibiotics were removed, the L-form bacteria regained the cell wall.
Dr. Mickiewicz believes that the ability of bacterial cells to go back and forth between normal cells and L-form bacteria is the key to the recurrence of some bacterial infections. “It is not so much antibiotic resistance as it is antibiotic evasion,” Dr. Mickiewicz explains. Still, the researchers don’t know how widespread this phenomenon is in bacterial cells. It could be that changing states, losing and regaining the cell wall, is just a natural part of the bacterial life cycle. It could be that this is constantly happening amongst all types of bacteria and we just haven’t looked before. “These results are very exciting,” comments Dr. Mickiewicz, “but we really just have more questions than answers.”
Future Work
Dr. Mickiewicz is planning several areas of future research. First, she wants to explore how using a combination of different antibiotics might affect bacterial species. Many antibiotics target the cell wall because it is a structure common to bacterial cells that is not found in human cells. By targeting the cell wall, clinicians know that the antibiotic will affect only bacterial cells and not human cells. However, as Dr. Mickiewicz’s research has shown, bacteria do have the ability to evade antibiotics targeting the cell wall by temporarily losing it. In future experiments, Dr. Mickiewicz wants to see how bacterial cells respond in the presence of cell wall targeting antibiotics in combination with other antibiotics that function by different mechanisms, for example by targeting bacterial membrane.
In addition, Dr. Mickiewicz plans to do experiments using samples from other groups of patients who are more susceptible to infectious diseases. For example,
cystic fibrosis
is a genetic disease that causes extra fluid to build up in the lungs. The extra fluid is the perfect place for bacteria to grow, so patients with cystic fibrosis are often given antibiotics—even if they don’t have an active infection—to prevent infections in the future. Since the antibiotics commonly given to patients with cystic fibrosis function by attacking the bacterial cell wall, Dr. Mickiewicz wants to find out whether L-form bacteria might reside in the lung fluid of these patients. She hypothesizes that she will find L-form bacteria, especially when tests for infection come back negative. “I really hope this research changes how we think about bacterial infections and antibiotics,” comments Dr. Mickiewicz.
Dr Katarzyna Mickiewicz is a Faculty of Medical Sciences Research Fellow at the Centre for Bacterial Cell Biology at Newcastle University in England. She is a trained immunologist who studies bacterial infections. When not in the laboratory, Dr. Mickiewicz enjoys traveling, hiking, and exploring the diversity of people and cultures in the world.
For More Information:
- Mickiewicz, K. et al. 2019. “Possible role of L-form switching in recurrent urinary tract infection.” Nature Communications, 10: 4379.https://eprint.ncl.ac.uk/260704
- Drage, L. K. et al. 2019. “Elevated urine IL-10 concentrations associate with Escherichia coli persistence in older patients susceptible to recurrent urinary tract infections.” Immunity & Ageing, 16: 1–11.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6625057/
- Kawai, Y., Mickiewicz K, and Errington J. 2018. “Lysozyme counteracts β-lactam antibiotics by promoting the emergence of L-form bacteria.” Cell, 172(5): 1038-49. https://eprint.ncl.ac.uk/246586
- Errington J. et al. 2016. “L-form bacteria, chronic diseases, and the origins of life.” Philosophical Transactions of the Royal Society of London, 371: 1701. https://eprint.ncl.ac.uk/229711
To Learn More:
- Errington lab.https://research.ncl.ac.uk/erringtonlab/
Urinary Tract Infections
- Urinary Tract Infection. U.S. Centers for Disease Control and Prevention.https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/uti.html
- Urinary Tract Infection (UTI). Mayo Clinic.https://www.mayoclinic.org/diseases-conditions/urinary-tract-infection/symptoms-causes/syc-20353447
- 6 Things You Should Know About UTIs in Older Adults. Cleveland Clinic.https://health.clevelandclinic.org/6-things-you-should-know-about-utis-in-older-adults/
Antibiotic Resistance
- Antibiotic Resistance. World Health Organization.https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- Antibiotic/Antimicrobial Resistance. U.S. Centers for Disease Control and Prevention.https://www.cdc.gov/drugresistance/about.html
- Antibiotics and Antibiotic Resistance. U.S. Food and Drug Administration.https://www.fda.gov/drugs/buying-using-medicine-safely/antibiotics-and-antibiotic-resistance
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. Katarzyna Mickiewicz

Prof. Jeff Errington, Drs. Katarzyna Mickiewicz and Yoshikazu Kawai
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

