| |
Highlights
Mitochondria are the powerhouses of the cell that produce
over 90% of the energy needed for our bodies to function properly.
These researchers discovered a mutation in the mitochondria of infants that was not previously described: a duplication of the ATAD3 sequence. Heart failure is a characteristic symptom of the ATAD3 duplication. Future research will focus on studying the function of the ATAD3 gene, which is involved in mitochondrial function and cholesterol metabolism.
Did you know that
our bodies produce about two billion mitochondria every second?
We need so many
mitochondria
for our cells to carry out all the functions that keep us alive. Mitochondria are so important because they are the site of energy production in our cells, where the food we eat is converted into energy for our cells and body to use. In addition, mitochondria are involved in sending messages between cells, cell death, calcium storage, and heat production, among other functions. For these reasons, mitochondria are known as the powerhouses of our cells.
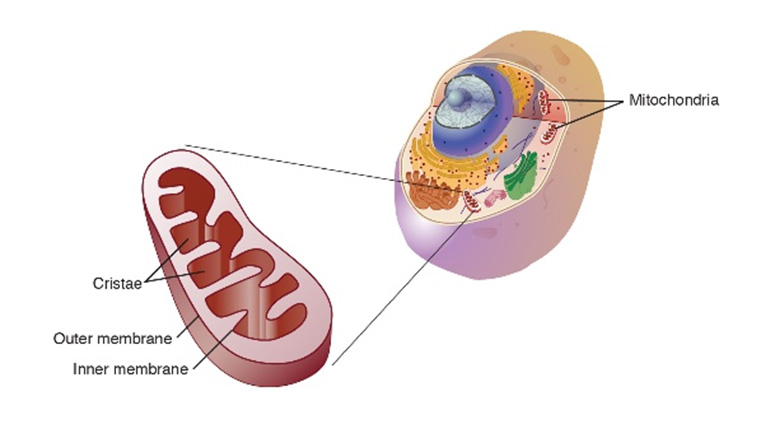
Figure 1. Mitochondria up close (left) and as part of the cell (right).
[Source: National Human Genome Research Institute.
https://www.genome.gov/genetics-glossary/Mitochondria]
When mitochondria do not work properly, the organs that require the most energy are typically affected. These include the brain, skeletal muscle, eyes, and heart. Genetic
mitochondrial diseases
occur in approximately 20 out of every 100,000 births. Scientists have identified
mutations
in at least 350 genes that can lead to mitochondrial diseases. When genetic mitochondrial diseases are severe enough, they can cause death in infants just hours, days, or weeks, after they are born. In about half of all such cases, parents may never receive a specific genetic diagnosis for their child.
Genetic analysis is important because it can allow parents to know their genetic risk for something similar happening again if they want to have other children or if the patients’ siblings want to have children in the future. In Australia, the Australian Genomics Health Alliance has been working to leverage the newest genetic technologies so families can receive a genetic diagnosis as quickly and efficiently as possible. The team that receives all such requests related to genetic mitochondrial diseases is led by Professor David Thorburn and Professor John Christodoulou at the Murdoch Children’s Research Institute in Melbourne, Australia. Both Profs. Thorburn and Christodoulou have dedicated their careers to improving the scientific understanding of genetic mitochondrial disorders.
When a request for a genetic diagnosis comes into the laboratory, the first step is to analyze the sample using a technique known as
whole genome sequencing,
which allows researchers to closely analyze the patient’s genetic information. As the leader of the Gene Discovery Group in Prof. Thorburn’s laboratory, Dr. Alison Compton is responsible for this analysis. About two years ago, Dr. Compton was analyzing such a sample when she noticed an odd sequence in a gene region known as ATAD3. An entire portion of the genetic sequence of ATAD3 was repeated. “I remember noticing this odd duplication,” recalled Dr. Compton, “but it wasn’t what I was looking for because this type of duplication had never been identified in previous patients with mitochondrial disease.”
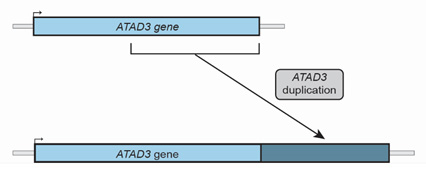
Figure 2. Normal ATAD3 gene (above) and ATAD3 gene with duplication (below).
[Source: Image courtesy of Dr. Compton and Dr. Frazier]
In conducting genetic analyses for other patients, Dr. Compton noticed several more patients in their cohort who had a similar duplication in the ATAD3 gene. She brought up this observation with her collaborator and colleague Dr. Ann Frazier, the lead researcher in mitochondrial diseases in cell and mouse models in Prof. Thorburn’s laboratory. Dr. Frazier had previously studied different genetic changes in ATAD3 and their link to mitochondrial disease. “There was clearly a pattern here,” Dr. Frazier commented. “The question was whether this was purely coincidence or whether it was clinically significant.”
To find out whether the duplication in ATAD3 was significant, Drs. Compton and Frazier began looking at the clinical symptoms of patients with and without the ATAD3 duplication. They found that patients with the ATAD3 duplication had remarkably similar symptoms, namely
heart failure
and death shortly before or after birth. This led the researchers to hypothesize that there might be a correlation between the ATAD3 duplication and the clinical presentation they were seeing in patients.
To test this hypothesis, the researchers first needed to be able to see the details of the specific genetic region. To do this, they used a technique called
polymerase chain reaction
or PCR. PCR allows for the amplification of a small portion of the genetic sequence using a single-stranded DNA segment known as a primer. Dr. Frazier created the primer specifically for amplification of ATAD3 through PCR. Using PCR, Drs. Compton and Frazier could clearly see the duplication of ATAD3.

Figure 3. Outside (left) and inside (right) of a PCR machine
[Source: https://commons.wikimedia.org/wiki/File:Pcr_machine3.jpg (left) and https://commons.wikimedia.org/wiki/File:Cycler_offen.JPG (right)]
Next, the researchers wanted to investigate whether the duplication of ATAD3 affected the protein that was created based on its genetic sequence. They used protein extracted from tissue samples donated by the patients’ families to determine that the duplicated ATAD3 gene did encode for a mutant protein. Further analysis showed this mutant ATAD3 protein affected its function, which is involved in mitochondrial function and cholesterol metabolism.
All this information was reported back to the families of the patients. The families also wanted to know if the genetic sequence found in the child was inherited from one or both parents. If the sequence was inherited, it would mean the parents would have a 25% chance of having a child with a similar disease in a future pregnancy. Using DNA samples provided by the patients’ parents, the researchers determined that, in each case, the duplication occurred spontaneously in the child and was not inherited from the parents.
When early data indicated a correlation between the ATAD3 duplication and heart failure, Dr. Compton and Dr. Frazier asked their colleagues around the world if they also had any patients with the ATAD3 duplication. They amassed a cohort of 17 patients who all had the same ATAD3 duplication and clinical symptoms. The symptoms of ATAD3 duplication were so characteristic that Drs. Compton and Frazier started to recognize it, just from knowing the symptoms. This was unusual compared to most of the other genes associated with mitochondrial disease, which often show variability in clinical symptoms, affected organs, and severity. Drs. Compton and Frazier continue to hear of more cases around the world with similar symptoms resulting from ATAD3 duplications.
At the same time, a separate
publication
described five more patients with ATAD3 duplications, making these the first descriptions of this particular ATAD3 mutation. Because it is rare for any two patients to have the exact same genetic mutation, it was surprising to Drs. Compton and Frazier to have identified 17 patients with the same ATAD3 duplication and heart failure.
In the future, Drs. Compton and Frazier plan to add more patients to their cohort of ATAD3 duplications and other mutations. They are also planning future studies into the function of the ATAD3 gene because little is known about it. To do this, the researchers are planning on creating knock-out cell lines where the gene for ATAD3 is removed from the cellular DNA to observe how cells can or cannot function without the protein.
Dr. Alison Compton leads the Gene Discovery group in the laboratory of Professor David Thorburn at Murdoch Children’s Research Institute in Melbourne, Australia. She specializes in the use of the newest scientific technologies for genetic sequencing and analysis. When not in the laboratory, Dr. Compton enjoys spending time with her family, reading and being outdoors.
Dr. Ann Frazier is a Postdoctoral Fellow in the laboratory of Professor David Thorburn at Murdoch Children’s Research Institute in Melbourne, Australia where she leads a research team studying mitochondrial disorders in cell and mouse models. She has spent her career studying the biology of mitochondrial diseases. When not in the laboratory, Dr. Frazier enjoys spending time with her family, gardening, biking, and swimming.
Professor David Thorburn is co-leader of Brain & Mitochondrial Research at the Murdoch Children's Research Institute in Melbourne, Australia. His research interests include the genetics of mitochondrial diseases and the improvement of methods for genetic diagnoses. For over 30 years, Professor Thorburn’s laboratory has been the national referral center for the diagnosis of mitochondrial diseases.
Professor John Christodoulou is co-leader of Brain & Mitochondrial Research at the Murdoch Children's Research Institute in Melbourne, Australia. He is a clinician who specializes in the diagnosis and management of children with mitochondrial and other rare diseases including Rett syndrome and phenylketonuria. His research interests include the development of improved diagnostics for these and other mitochondrial diseases.
For More Information:
- Frazier, A. et al. 2020. “Fatal Perinatal Mitochondrial Cardiac Failure Caused by Recurrent De Novo Duplications in the ATAD3 Locus.” Med 2(1): 49-73. http://dx.doi.org/10.1016/j.medj.2020.06.004
- Frazier, A., D. Thorburn, and A. Compton. 2019. “Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology.” Journal of Biological Chemistry, 294(14): 5386-95.https://pubmed.ncbi.nlm.nih.gov/29233888/
- Desai, R. et al. 2017. “ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism.” Brain, 140: 1595-1610. https://pubmed.ncbi.nlm.nih.gov/28549128/
To Learn More:
- Mitochondrial Genetic Disorders. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/7048/mitochondrial-genetic-disorders
- Types of Mitochondrial Disease. United Mitochondrial Disease Foundation. https://www.umdf.org/what-is-mitochondrial-disease/types-of-mitochondrial-disease/
- Australian Genomics Health Alliance. https://www.australiangenomics.org.au/
- Mitochondria-Fundamental to Life and Health. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684129/
- Mitochondria. National Human Genome Institute.https://www.genome.gov/genetics-glossary/Mitochondria
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. Alison Compton

Dr. Ann Frazier

Professor David Thorburn

Professor John Christodoulou

The Brain and Mitochondrial Research Group led by Professors John Christodoulou and David Thorburn
Back, left to right: Sabrina Kaminsky, Sumudu Amarasekera, Dr. Nicole Van Bergen, Dr. Shalini Thirukeswaran, Simone Tregoning, Dr. Rocio Rius, Professor David Thorburn, Professor John Christodoulou, Tegan Stait, Dr. Ann Frazier, Sean Massey, Dr. Alison Compton, Dr. Ken Pang, Nicole Andrews, Dr. Xiaomin Dong
Front (kneeling), left to right: Yau Chung Low, Cameron McKnight, Dr Simran Kaur

Watch Professor Thorburn discuss the ATAD3 duplication

Hear from one of the families affected by the ATAD3 duplication
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

