Highlights
Clinical trials are conducted to ensure treatments and vaccines are safe and effective. The purpose of vaccination is to prepare the immune system to fight infection in the future.
Clinical trials are divided into three phases: Phase I, Phase II, and Phase III. Phase I determines whether a potential new treatment is safe; Phase II determines if the treatment is effective; and Phase III determines if the new treatment is better than treatments already available. For the COVID-19 vaccine, Phases II and III were combined because there were no other vaccines available to use as a comparison.
These researchers conducted clinical trials of Moderna’s COVID-19 vaccine for adolescents ages 12-17 years. The data they collected showed that the vaccine had an acceptable safety profile, generated strong immune responses, and showed efficacy of 93% in preventing COVID-19 symptomatic disease in the adolescent population. Current clinical trials are now studying the safety of this vaccine in younger children (6 months to 11 years).
As you probably know, we have been living through a historic
pandemic
caused by the spread of the virus known as
SARS-CoV-2
that causes the infection
COVID-19.
SARS-CoV-2 is a highly transmissible virus that spreads from person to person through droplets released into the air when an infected person breaths, coughs, or sneezes.
Figure 1. SARS-CoV-2 virus
[Source: https://phil.cdc.gov//PHIL_Images/23311/23311_lores.jpg]
Once in the body, the SARS-CoV-2 virus begins to replicate, causing symptoms such as fever, headache, shortness of breath, loss of taste and/or smell. Our
immune system
does its best to fight the infection, but sometimes its best efforts are not enough.
According to data from Johns Hopkins University, over 5 million people have died from COVID-19 infections worldwide, including over 800,000 people in the U.S. As the SARS-CoV-2 virus continues to spread, it
mutates
into different
variants,
including
Delta
and
Omicron,
and the death toll due to the pandemic is increasing daily.
One of the best tools we have to fight the pandemic on a global scale is vaccines that can protect against COVID-19 infection and slow transmission of the virus. In the United States, three vaccines are currently in use to prevent COVID-19 infection. These vaccines are made by Pfizer, Moderna, and Johnson & Johnson. The Pfizer vaccine has received full approval from the U.S. Food and Drug Administration (FDA); the Moderna and Johnson & Johnson vaccines have received Emergency Use Authorization from the FDA and are currently waiting for a decision regarding full approval.
To better understand the process of ensuring the safety and efficacy of these new vaccines, What A Year! spoke with Dr. Katherine Luzuriaga, Professor of Pediatrics and Molecular Medicine and Vice Provost of Clinical and Translational Research at the University of Massachusetts Chan (UMass Chan) Medical School and Director of the University of Massachusetts Center for Clinical and Translational Science. Dr. Luzuriaga is a renowned expert on pediatric vaccine development and is currently leading the clinical trials in children and adolescents for the Moderna vaccine at UMass Chan in Worcester, MA. Dr. Luzuriaga spoke about the process of vaccine development, how clinical trials ensure vaccine safety, and specific details about the clinical trials she oversees.
Vaccines and COVID-19
The purpose of vaccination is to prepare the immune system to fight infection in the future. The immune system has many different cells that do the best they can when they encounter a new pathogen. However, it takes time for the body to mount a full response if the pathogen is previously unknown. Vaccines work by preparing the immune system in advance for a new pathogen, so one’s body can be ready to fight the infection quickly and avoid severe illness.
Most vaccines currently in use are made from small pieces of the pathogen causing disease. The vaccines to prevent COVID-19 developed by Pfizer and Moderna are distinct because they are made using a new vaccine technology called messenger ribonucleic acid vaccines or
mRNA
vaccines. mRNA is a single strand of genetic material that takes a copy of a gene from inside the nucleus of the cell to the site of protein production in the cell.
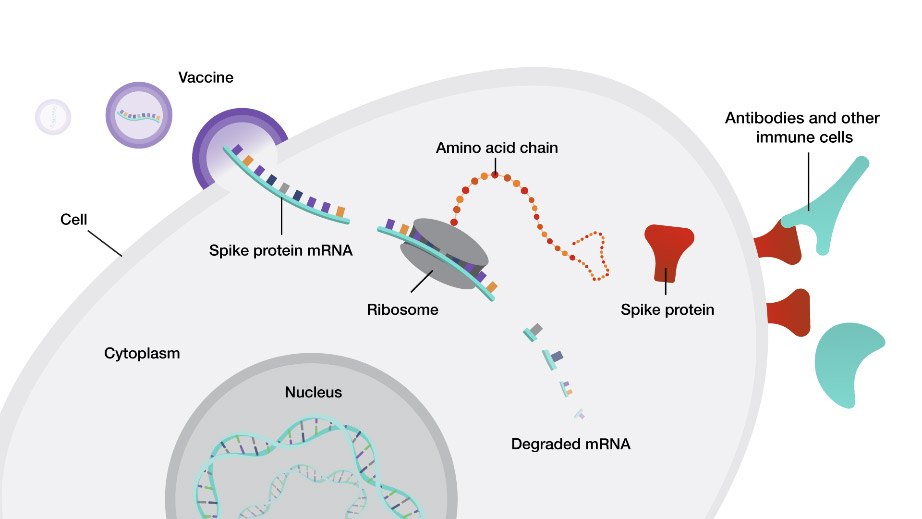
Figure 2. How mRNA vaccines work. The vaccine delivers mRNA from the SARS-CoV-2 spike protein made in the laboratory to cells. The mRNA uses cellular machinery to produce the spike proteins so that the immune system can develop antibodies against them. In the future, when the immune system is exposed to SARS-CoV-2, it can recognize the virus and be more equipped to fight the infection.
[Source: https://www.genome.gov/about-genomics/fact-sheets/Understanding-COVID-19-mRNA-Vaccines]
mRNA vaccines use laboratory-made genetic material to teach the immune system how to make a certain protein without the need for any piece of the real virus. The body degrades mRNA after a few days; the lack of mRNA persistence lessens the likelihood of long-term side effects of the vaccine. For the COVID-19 vaccine, researchers have used different parts of the proteins found on the spikes of the SARS-CoV-2 virus as the genetic material.
The FDA and Clinical Trials
The FDA is a regulating body of the U.S. government that is responsible for reviewing the data regarding the safety and efficacy of all new medicines, treatments, and devices. In most cases, new treatments are not made available to the general public until they have received FDA approval. However, the FDA can make exceptions in the case of public health emergencies, such as the ongoing COVID-19 pandemic. In these exceptional cases, the FDA can grant Emergency Use Authorization based on preliminary studies while more data are being gathered.
When the FDA decides to grant Emergency Use Authorization or full approval for a new treatment, a panel of experts reviews the submitted data. The data include testing for safety and efficacy in cells and animal models in the laboratory, as well as testing done in humans. Research conducted to test new treatment methods in humans is called a
clinical trial.
Clinical trials are governed by strict ethical and safety rules and overseen by the FDA.
Clinical trials are divided into three phases: Phase I, Phase II, and Phase III. In Phase I clinical trials, researchers seek to determine the best dose of a vaccine to give to patients. They are looking for a dose that elicits good immune responses while minimizing side effects. Trials begin with a low dose and monitor participants for any side effects. If a low dose of the treatment is shown to be safe, the dose is slowly increased until researchers determine the highest possible dose that can be given without adverse side effects. If a treatment seems to cause severe side effects or is otherwise unsafe, the clinical trial stops at Phase I and does not move forward.
If the safety of the new treatment can be shown in Phase I clinical trials, the treatment can move on to Phase II. In Phase II, researchers seek to determine whether the treatment is effective. If the treatment is deemed effective in Phase II, it can move on to Phase III.
In Phase III, researchers determine whether the treatment in question is better than treatments that are already available. New treatments for a given disease or condition for which other treatments already exist must be shown to be better than existing treatments in order to receive FDA approval. In the case of vaccines for COVID-19, this is a totally new treatment for a never-before-seen disease, so Phases II and III of the clinical trials can be combined.
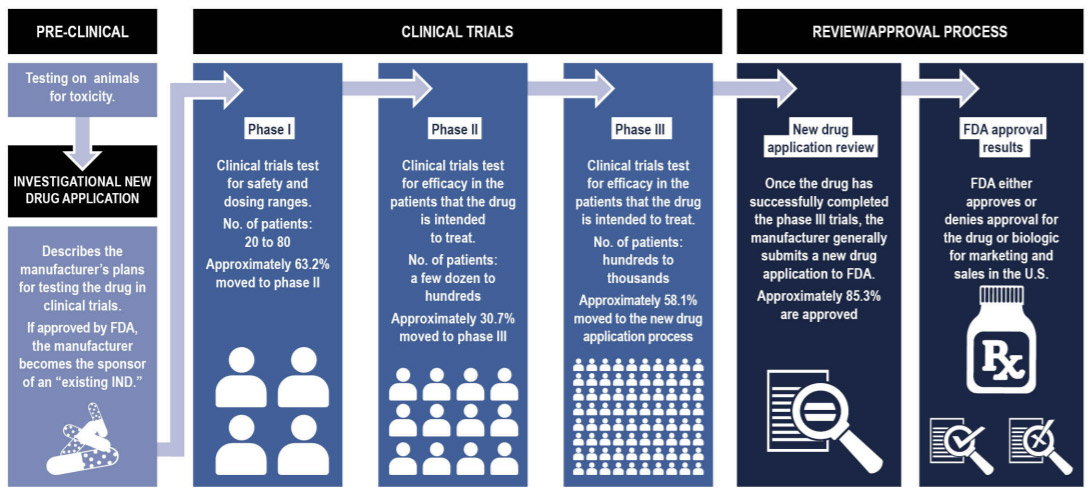
Figure 3. Typical process for new drugs and devices to be approved by the Food and Drug Administration
[Source: Government Accountability Office analysis of data from the Food and Drug Administration and a 2016 collaborative study by Biotechnology Innovation Organization.
https://www.gao.gov/assets/gao-17-564.pdf]
In order to receive Emergency Use Authorization, data from clinical trials of each of the three vaccines had to show that they were safe in humans and effective at preventing COVID-19 infection. These clinical trials were conducted in adults over 18 years of age.
Clinical Trials in Children
Once the vaccines received Emergency Use Authorization in adults, researchers began to conduct clinical trials in teenagers and children. This process is being carried out very slowly and carefully to ensure the safety of everyone involved. “This is important because children are not just miniature adults,” explained Dr. Luzuriaga. “The organs and bodies of children develop and mature at different times.” In addition, children, especially younger children, may not be able to tell us about potential side effects in the same way as adults.
Clinical trials in those under 18 years have been divided into four distinct age groups: 12-17 years, 6-11 years, 2-5 years, and 6-23 months. The Moderna TeenCOVE study is researching safety and efficacy of the Moderna COVID-19 vaccine in adolescents 12-17 years of age. The Moderna KidCOVE study is researching the safety and efficacy of the COVID-19 vaccine in children under 12 years old. These studies are carried out in age order from oldest to youngest, starting with adolescents ages 12-17 years.
The TeenCOVE study was conducted at 25 medical research centers across the country, including at UMass Chan where Dr. Luzuriaga is the lead researcher. “We are so proud to be chosen as one of the research sites for this crucial vaccine with the potential to protect the health of our children and families,” remarked Dr. Luzuriaga. “We take this responsibility very seriously.”
The TeenCOVE study began recruiting teenage participants in December 2020 and is estimated to be completed around June 2022. In order to participate, adolescents had to be in good health. The trial excluded participants who had previously tested positive for COVID-19; had traveled outside of the United States in the month prior to their participation in the study; had participated in another trial in the past month; or were a current smoker or had a history of smoking.
The purpose of the study was to determine three things: the safety of the vaccine;
immunogenicity,
meaning the strength of the immune system’s response the vaccine produced; and efficacy at preventing COVID-19 infection.
In total, 3,732 adolescents enrolled in the TeenCOVE study, in which teens received the same dose shown to be safe and effective in adults. TeenCOVE’s goal is to determine the immunogenicity of the vaccine and its effectiveness at preventing COVID-19 infection in the study population (adolescents ages 12-17).
The researchers divided study participants into two groups: one group received the COVID-19 vaccine candidate, and the other group received a
placebo.
Everyone received a series of two injections 28 days apart. The group that received the COVID-19 vaccine candidate consisted of 75% of the participants while the remaining 25% of participants received the placebo. The study was conducted blinded, meaning that the participants and the investigators did not know who received which treatment. All participants knew there was a chance they might not be getting the real vaccine, but no participant knew for sure whether they had gotten the vaccine or the placebo.
The clinical trial was designed so that all participants would receive close monitoring by a team of medical professionals. Participants and their caregivers had telemedicine visits with study doctors after each injection, and monthly telemedicine visits after that. In addition, caregivers of participants were asked to use an app on their smartphone to record any symptoms of COVID-19 each day. If COVID-19 symptoms are reported, the team of medical professionals carefully monitors the patient’s symptoms and course of infection.
In May 2021, Moderna reported data from Dr. Luzuriaga’s team together with data from other research sites around the country and showed that the vaccine stimulated an immune response in adolescents comparable to that of adults. Using the U.S. Centers for Disease Control and Prevention’s definition of COVID-19 infection, researchers calculated the efficacy of the Moderna vaccine to be 93% in the adolescent population.
In general, the results of the clinical trial show that the vaccine was well tolerated with few side effects. The most common side effects were pain at the injection site, headache, chills, fatigue, and muscle aches. Participants will continue to be monitored for 12 months to determine the effectiveness of the vaccine over time.
As soon as the data from the Phase I TeenCOVE clinical trial showed the vaccine was safe in adolescents, Dr. Luzuriaga and her colleagues across the country began Phase I clinical trials for the KidCOVE study in children 6-11 years, and then in children under 5 years of age.
In total, over 13,000 children and their families have volunteered to participate in the KidCOVE clinical trial of the Moderna vaccine across the three age groups. The clinical trial is expected to be completed for all age groups around June 2023.
Dr. Luzuriaga wants to reassure children, adolescents, and their parents that available data indicate that the COVID-19 vaccines are safe and effective. Vaccines are one of our best tools to prevent disease, break the chain of infections, and bring the pandemic to an end. “Our clinical trials are highly rigorous and carefully monitored, so we know that our results are reliable,” commented Dr. Luzuriaga. “All of this is possible thanks to the thousands of children and their parents who have chosen to participate in this research, which we know will have a global impact.”
One common concern about the vaccine, in both adults and children, is that they were developed too quickly to be safe. While it is true that the specific vaccines for COVID-19 were developed quickly, this is because scientists were able to take advantage of the research conducted by hundreds of scientists who had been working on vaccine development for decades. The prototype for what would become the mRNA platform used by the Pfizer and Moderna COVID-19 vaccines began development in the 1990’s. “We are fortunate that the availability of the mRNA technologies has allowed the rapid development of safe and effective COVID vaccines,” added Dr. Luzuriaga.
Dr. Luzuriaga emphasizes that it is critical that everyone who is eligible to receive a COVID-19 vaccine do so as quickly as possible. “The clinical trials in adults and adolescents have shown us that the Moderna COVID-19 vaccine is safe and effective. We don’t have full results yet for younger children under 12 years, and we will make recommendations based on what the data show.”
Dr. Katherine Luzuriaga is Professor of Pediatrics and Molecular Medicine and Vice Provost of Clinical and Translational Research at the University of Massachusetts Chan Medical Center and Principal Investigator and Director of the University of Massachusetts Center for Clinical and Translational Science. Dr. Luzuriaga is currently leading the clinical trials in children and adolescents for the Moderna vaccine at the University of Massachusetts Chan Medical Center in Worcester, MA. When not in the laboratory, Dr. Luzuriaga likes cycling, kayaking, yoga, and trying new recipes with family and friends.
For More Information:
- Spencer, SEW. UMass Medical School to start trial of Moderna COVID-19 vaccine in teens. January 2021. https://www.umassmed.edu/news/news-archives/2021/01/umass-medical-school-researchers-to-start-trial-of-moderna-covid-19-vaccine-in-teens/
- TeenCOVE Clinical trial.https://clinicaltrials.gov/ct2/show/NCT04649151
- KidCOVE Clinical Trial. https://clinicaltrials.gov/ct2/show/record/NCT04796896?term=NCT04796896&rank=1
- 1. Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, Ding B, Dooley J, Girard B, Hillebrand W, Pajon R, Miller JM, Leav B, McPhee R. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med. 2021 Dec 9;385(24):2241-2251. doi: 10.1056/NEJMoa2109522. Epub 2021 Aug 11. PMID: 34379915; PMCID: PMC8385554.https://pubmed.ncbi.nlm.nih.gov/34379915/
To Learn More:
COVID-19 Vaccines
- Centers for Disease Control and Prevention.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
- U.S. Food and Drug Administration.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- World Health Organization.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines
Clinical Trials
- Moderna COVE Study.https://www.modernatx.com/cove-study
- Moderna TeenCOVE Study. https://connect.trialscope.com/studies/a6dd51e8-c0a0-4ad5-b80b-9b4cda3c0e36
- Moderna KidCOVE Study.https://trials.modernatx.com/study/?id=mRNA-1273-P204
- American Cancer Society.https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/phases-of-clinical-trials.html
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

