Highlights
Malaria is a mosquito-borne disease responsible for an estimated 249 million cases and 608,000 deaths worldwide in 2022. Currently available vaccines for malaria will have an impact on public health but have significant limitations.
These researchers sought to develop an mRNA vaccine for malaria to address the limitations of current malaria vaccines. This vaccine was shown to be effective at preventing malaria in mouse models.
In future work, the researchers will continue to optimize the malaria vaccine by testing it in human cells and non-human primate models. They hope that one day their research will lead to more effective malaria vaccines that can save lives worldwide.
Did you know that in 2023 nearly half of the world’s population was at risk for
malaria?
Malaria is caused by mosquito-borne parasites and is widespread in tropical climates. Symptoms of malaria can range from mild fever, chills, and headache to life-threatening seizures and difficulty breathing. In 2022, there were an estimated 249 million cases of malaria that caused 608,000 deaths. Children under 5 years old, pregnant women, and people with
HIV
are at highest risk of severe infection.
Vaccines
are the best way to protect against malaria infection, and two malaria vaccine options are now available. However, these vaccines have significant limitations. “It is great that we have a malaria vaccine available,” commented Dr. Lauren Holz, Research Fellow in
Microbiology
and
Immunology
in the laboratory of Professor William Heath at the University of Melbourne and the Peter Doherty Institute for Infection and Immunity (Doherty Institute) in Australia, “and there is still room for improvement.” Dr. Holz is a liver immunologist who is part of a team that is developing a new vaccine that the researchers hope will be more effective than those currently available. Recent studies by Dr. Holz and colleagues show that their vaccine technology is effective at preventing malaria infection in mouse models.
The Life Cycle of Malaria
To better understand this research, let’s first learn about malaria transmission and the
immune system.
The life cycle of the malaria parasite occurs in both female Anopheles mosquitoes and the humans they infect. When a person gets bitten by a malaria-infected mosquito, the mosquito injects immature parasites into the skin. These immature parasites travel over several hours from the skin to the liver, where they infect
hepatocytes,
the primary type of liver cell. Once the immature parasites are in the liver, they reproduce and grow into mature parasites. The mature parasites are released from the liver where they infect red blood cells. Malaria symptoms appear when mature malaria parasites infect red blood cells.
If a person infected with malaria gets bitten by a mosquito that is not yet carrying the malaria parasite, the parasite will enter the mosquito through the blood. In the mosquito, the malaria parasite undergoes another life cycle that enables the mosquito to infect another human. This is how malaria spreads.
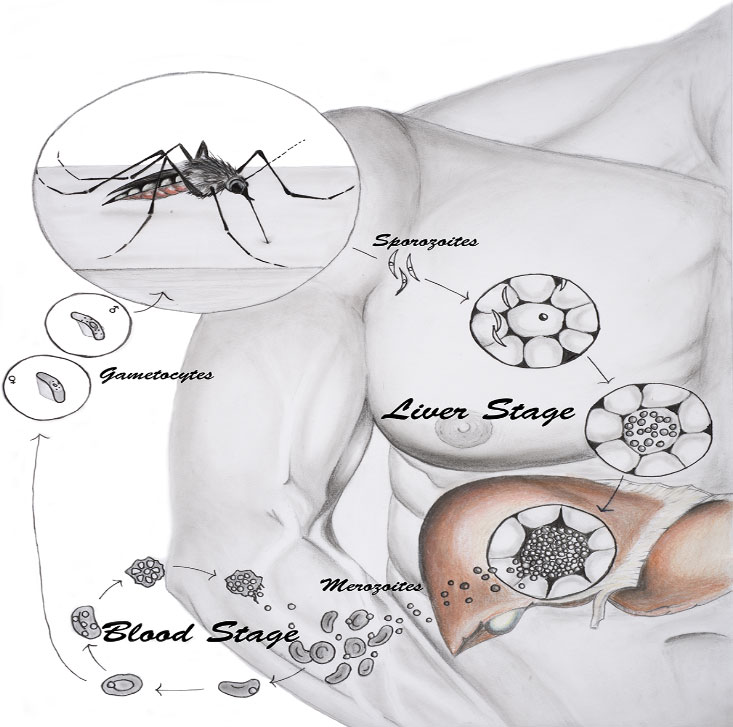
Figure 1. Life cycle of malaria. When an infected mosquito bites a human, it releases immature parasites (sporozoites) into the skin. The sporozoites travel to the liver (liver stage) where they mature. Mature parasites (merozoites) are released into the blood (blood stage) where they can cause symptoms and be transmitted to uninfected mosquitoes.
[Source: Holz, L. et al, 2016, Fig 1]
The Immune System and Vaccines
The immune system is a network of cells, tissues, and organs in the body that prevent infection and fight disease. Each type of immune cell and organ plays a different role, and they all work together to keep the body healthy. When a new
pathogen,
such as the malaria parasite, enters the body, the immune system must recognize the pathogen as foreign and mount a response against it. If this happens quickly and strongly, the immune system can fight off the pathogen before you feel any symptoms. If this happens more slowly or less strongly, you may experience symptoms of disease as your body continues fighting the pathogen. Once the infection has passed, certain immune cells, called
resident memory T cells,
remain in the tissue and remember the pathogen so it can be fought more effectively in the future.
The purpose of a vaccine is to enable the immune system to produce an immune response before the body becomes infected. This allows the body to identify and fight infection before you get sick. There are many types of vaccines that use different mechanisms to introduce the immune system to a pathogen. Researchers select the vaccine platform they think will work best based on the type of pathogen and how the pathogen affects the body.
The First Malaria Vaccines
The World Health Organization approved the first malaria vaccine, RTS,S, in 2021 and a second vaccine, R21, in October 2023. These vaccines are recommended for children living in areas with high malaria transmission just before peak season. The World Health Organization expects these two vaccines to have an important public health impact.
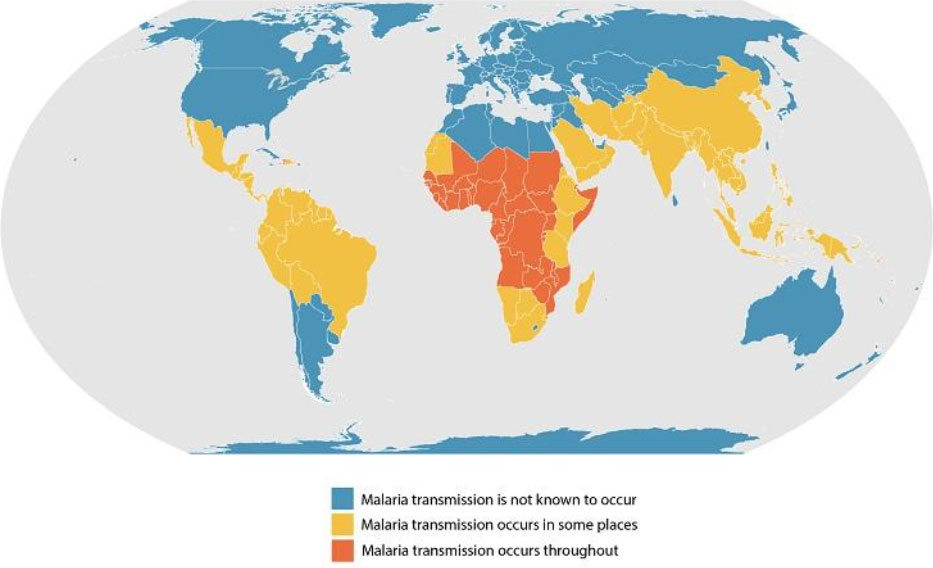
Figure 2. Map of known malaria transmission rates around the world. Areas with some transmission are shown in yellow and areas with highest transmission are shown in orange.
[Source: https://www.cdc.gov/malaria/about/distribution.html]
These malaria vaccines have several important limitations. First, the vaccine targets the malaria parasites as they travel from the skin to the liver. This is a very short window of time to eradicate the parasites. If the parasites make it to the liver before a full immune response can be mounted, the vaccinated person will still become sick. Second, the vaccine is not effective against all strains of the malaria parasite. If you are vaccinated and become sick with a disease strain that is different from the vaccine, you won’t be protected from the disease. Third, the vaccine does not work to prevent malaria in people who have already been exposed to the disease. In regions where malaria is widespread, this is most of the adult population. For this reason, the malaria vaccines currently available are only recommended for children.
An mRNA Vaccine for Malaria
Dr. Holz and colleagues have been working for years to develop a new malaria vaccine that reduces these limitations. First, this novel vaccine specifically targets the malaria parasites in the liver, the only time in the malaria life cycle when the parasites are all contained in a single organ. Second, the researchers chose the large ribosomal subunit protein L6 (RPL6) as the vaccine target because it is found in all strains of malaria parasites. This reduces variability in the effectiveness of the vaccine due to multiple strains of malaria infection. Finally, Dr. Holz and colleagues made it their goal to stimulate such a strong immune response from the vaccine so that it could be effective regardless of prior malaria exposure. This would mean the vaccine could be available for adults and children alike.
The rapid development of vaccines against SARS-CoV-2, the virus that causes COVID-19 infection, using
mRNA vaccine
technology inspired Dr. Holz and colleagues to use a similar technique for their malaria vaccine. mRNA vaccine technology is so powerful because it allows the immune system to receive information about an entire protein, not just a piece of the protein. Dr. Holz and colleagues applied their knowledge of malaria vaccines to the mRNA vaccine platform with the goal of developing an mRNA vaccine for malaria.
These researchers have performed many experiments to test the efficacy of their malaria mRNA vaccine system in mouse models. They knew from previous research that tissue resident memory CD8 T cells were key to mounting an immune response to malaria in the liver. The researchers tested several combinations of RPL6 with an additional molecule α-galactosylceramide (αGC) known to activate another immune cell population known as Natural killer T (NKT) cells. They found that the combination of RPL6 plus αGCB protected mice from subsequent malaria infection in 80% of cases. These numbers were further improved by a vaccine boost after 30 days.
Finally, the researchers compared the effectiveness of their vaccine in mice previously exposed to malaria infection and controls. They found the vaccine protected the mice from infection whether or not they had previously been exposed to malaria.
“These results are promising,” commented Dr. Holz, “but we are still many years away from a vaccine that could be given to humans.” The experiments described here were done in mice using a strain of malaria that affects mice. Next, the researchers plan to conduct similar experiments in humanized mice using a strain of malaria that affects humans. If those results are successful, the next steps would include testing the vaccine on human blood samples and in non-human primates. If all of this testing shows that the vaccine is effective, the next step would be clinical trials to evaluate the vaccine’s safety and effectiveness in humans.
One limitation of the current vaccine model is that it must be kept refrigerated at 4 degrees Celsius. This can pose a challenge when vaccinating large numbers of people in hot areas without consistent access to refrigeration. For that reason, collaborators in New Zealand are working to develop a version of the malaria mRNA vaccine that can be freeze-dried and reconstituted on site. This would eliminate the need for refrigeration. “We are hopeful that the next generation of malaria vaccines will have an even greater public health impact than the first,” Dr. Holz concluded.
Dr. Lauren Holz is a Research Fellow in Microbiology and Immunology in the laboratory of Professor Bill Heath at the University of Melbourne and the Doherty Institute in Melbourne, Australia. She is a specialist in liver immunology and her research focuses on the development of vaccines to prevent malaria. When not in the laboratory, Dr. Holz enjoys spending time at the beach with her friends and watching trashy TV.
Dr. William Heath is Professor in the Department of Microbiology and Immunology at the University of Melbourne and the Doherty Institute in Melbourne, Australia. His research focuses on the immune response with the goal of furthering our understanding of malaria and developing improved vaccination strategies. When not in the laboratory, Dr. Heath enjoys playing golf and hanging out with his family and friends.
For More Information:
- Ganley, M. et al. 2023. “mRNA vaccine against malaria tailored for liver-resident memory T cells.” Nature Immunology, 24(9): 1487-1498. https://pubmed.ncbi.nlm.nih.gov/37474653/
- Holz, L. et al. 2016. “Protective immunity to liver-stage malaria.” Clinical and Translation Immunology, 5(10): e105. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5099428/
To Learn More:
- Heath Laboratory. https://biomedicalsciences.unimelb.edu.au/sbs-research-groups/microbiology-and-immunology-research-groups/
- World Health Organization. https://www.who.int/health-topics/malaria#tab=tab_1
- Vaccines and Immunization. https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1
- Vaccine Types. https://www.hhs.gov/immunization/basics/types/index.html
- mRNA Vaccines. https://medlineplus.gov/genetics/understanding/therapy/mrnavaccines/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

