Nearly every product you buy in the grocery store comes with an expiration date—even water! These dates are regulated by the Food and Drug Administration (FDA) to protect consumers from eating expired foods that might make us sick. Let’s take milk as an example. Even refrigerated, milk will only last a certain amount of time—usually one to two weeks—before spoiling. You can extend the life of milk by putting it in the freezer, but if you try to defrost the milk it won’t taste like milk anymore. The low temperatures of the freezer alter the composition of the milk irreversibly. What if there was a way to freeze milk and have it come back to its liquid state tasting like fresh milk? This would certainly cut back on waste by decreasing the amount of expired milk that is thrown out or dumped down the drain. There is a plentiful supply of milk, though, so this is not an intensive area of scientific research.
Food products are not alone in having an expiration date. It turns out that our
organs
do too. Organs that would last a lifetime inside us survive only a few hours outside the body. This is a major issue for patients in need of
organ transplants.
According to the
United Network for Organ Sharing
today there are 116,121 patients waiting for an organ.
The supply of viable organs is not enough to meet the demand because, in many cases, organs cannot be preserved for long enough to get them from the donor to the recipient for transplant. Extending the shelf life of human organs, therefore, is an important area of medical research that has life and death consequences for thousands of patients.
There are many possibilities for how organs might be preserved. In 1984, a group of researchers at the University of Wisconsin developed a chemical solution that helped prevent damage from cold temperatures while organs were stored on ice. This breakthrough dramatically changed the field of organ transplantation by extending the life of organs outside the body from minutes to hours (about 3 hours for heart, 12 hours for liver, 20 hours for kidney). This is still the method most commonly used today.
Lengthening the amount of time an organ can be preserved would be a huge advancement in organ transplantation. However, being able to preserve organs for a longer period of time is very difficult. Exposure to cold temperatures for extended periods of time can cause irreversible tissue damage that renders the organ unsuitable for transplantation. Even if the organ survives the cold temperatures, thawing presents its own challenges to organ function. The next two What A Year articles will present researchers who are addressing the issue of organ preservation from different angles:
supercooling
live organs and thawing frozen organs. This month, we highlight the work from the Center for Engineering in Medicine at Massachusetts General Hospital and Harvard Medical School. There, the team led by PhD student and Medical Fellows Tim Berendsen, Bote Bruinsma and faculty member Dr. Uygun have developed a novel
protocol
that extended the shelf life of rat livers from 24 hours to 72 hours, triple what current technology can achieve. This extended time means more livers will be able to be preserved.
Preservation Protocol
The protocol centers around the concept of supercooling. Supercooling occurs when cells are cooled to temperatures below freezing without actually becoming frozen. In the supercooled rat livers that Dr. Uygun used in his experiments, the organs remain alive at freezing cold temperatures yet unfrozen. The supercooling process is facilitated with cold temperatures and chemicals that prevent cell damage in extremely cold temperatures. The protocol has three distinct phases for the treatment and storage of rat livers: loading of preservative agents, supercooling, and recovery. Recovered rat livers were then transplanted into healthy rats who were monitored for liver health. Let’s look at each of these phases.
Phase 1: Loading
Since exposure to cold temperatures can result in organ damage, Dr. Uygun and his team sought to develop effective methods to protect cells from the cold. They began to modify the chemical solution developed in 1984, and searched for compounds that would help protect cells as temperatures dropped. Through a series of experiments using individual liver cells, the researchers identified 3-O-methyl-3-glucose or 3-OMG as the best compound for their purposes. 3-OMG is a
synthetic
compound similar to glucose. It is recognized by cells and allowed to enter through the cell membrane, but it cannot be used by cells. As a result, the compound builds up inside the cells and helps prevent damage due to cold temperatures.
In addition to 3-OMG, the researchers added polyethylene glycol to the solution. Polyethylene glycol was shown to protect cell membranes from cold damage. It is thought to work by stabilizing cellular membranes and preventing swelling during storage.
The chemical solution the researchers developed was tested using livers in a process known as machine
perfusion.
Perfusion is the process of pumping blood throughout the body. Since a donated organ is no longer perfused with blood, this process is simulated by a machine, known as machine perfusion, which includes a solution, called perfusate, that is similar to blood in nutrients and oxygen.
Immediately after recovery from the donor, the organ was connected to external equipment that provided 60 minutes of machine perfusion at room temperature, which enabled loading 3-OMG into the liver cells and prepared the cells for supercooling.

Figure 1. Liver and machine perfusion apparatus.
Picture Credit: Bote Bruinsma
Phase 2: Supercooling
After 60 minutes of machine perfusion at room temperature, the liver was detached from the perfusion machine, flushed with a chemical solution containing 3-OMG and polyethylene glycol in addition to the 1984 solution at 4 degrees Celsius (about 39 degrees Fahrenheit). The bag was sealed and submerged into antifreeze and cooled at a rate of 1 degree Celsius every ten minutes until it reached -6 degrees Celsius (about 21 degrees Fahrenheit). Previous experiments showed that this was the lowest temperature at which the liver would remain supercooled, that is cold but unfrozen. The liver was stored at this temperature for 72 to 96 hours.
Phase 3: Recovery
After storing the supercooled livers for 72 to 96 hours, it was time to bring them back to room temperature and prepare them for transplantation. The temperature of the livers was slowly raised at a rate of about 1 degree Celsius per minute until it returned to 4 degrees Celsius. The liver was then reattached to machine perfusion for three hours while the recipient animal was prepared for transplantation.
Measuring Success
Following transplantation, the recipient animals were monitored for up to three months to ensure survival. Blood samples were taken frequently to check for any signs of liver disease or damage.
After three months, all the animals who had received a transplanted liver stored for 72 hours were still alive. Of the animals that received livers stored for 96 hours, 58% survived. None of the surviving animals showed any signs of developing liver problems, demonstrating the supercooled livers were able to function normally for at least three months following transplantation.
In order to further test the success of their protocol, the researchers repeated these same experiments several times with one of four key components missing: 3-OMG, polyethylene glycol, supercooling, and machine perfusion. The team observed that without either 3-OMG or polyethylene glycol, the animals did not survive more than one week after transplantation. Without supercooling or machine perfusion, the animals did not survive even one hour after transplantation. These results further demonstrate the importance of the entire protocol to the success of these experiments and liver transplantation. All of the steps are necessary to ensure the best results.
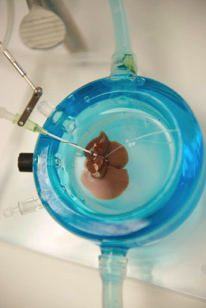
Figure 2. Supercooled liver
Picture Credit: Bote Bruinsma
Future Work
Through multiple intensive experiments, the team has shown that rat livers can be preserved for 3 to 4 days at freezing temperatures and still function as liver transplants for at least three months. This is a remarkable achievement in the field of organ preservation and provides the basis for future work that can expand these results.
The ultimate goal is to allow the prolonged preservation of human organs for transplant into sick patients in need of donated human organs. There is still much work to be done before the results from these experiments can be translated into humans.
With regards to the organ itself, rat and human livers are significantly different, and human livers may present additional challenges to this protocol. For example, the amount of liquid that must be prevented from freezing in human livers is significantly greater than that of rats. In addition, further research is needed with regards to specific liver functions and the potential development of liver complications after transplantation. Similarly, adjustments must be made for similar procedures to be adopted for other organs such as the heart or kidneys.
Dr. Uygun is interested in pursuing several applications of this research in the field of organ transplantation. First, they are currently involved in experiments to test additional chemical additives or other adjustments to the protocol to improve survivability and liver functionality. Second, they plan to further study each step in this protocol to better understand how and why it works. This line of questioning can help lead to better treatments for liver disease in addition to improved methods of liver transplantation. Finally, this study focused on the transplantation of healthy livers that had been preserved. However, the majority of donated livers have some sort of injury, such as those donated by a person who has experienced death from a heart attack. A long-term goal is to figure out how supercooling might improve the functionality of damaged organs for use in transplantation.
Dr. Tim Berendsen is a surgical resident in the Netherlands.
Dr. Bote Bruinsma is Associate Director of Medical Affairs at Axovant Sciences in New York City.
Dr. Korkut Uygun is Assistant Professor of Biomedical Engineering at Harvard Medical School, Founding Director of the Cell Resource Core at Massachusetts’s General Hospital, and the deputy Director of Research at Shriners Hospitals for Children, Boston. His work in the field of organ transplantation has focused on the development of novel methods for organ preservation and assessment, which includes machine perfusion technologies and protocols such as the one described above, as well development of quantitative biomarkers to determine viability of non-ideal organs for use in transplantation. Outside of the laboratory Dr. Uygun likes to read economy and tech blogs, play computer games and work on math and science projects with his daughter.
For More Information:
- Berendsen, T., et al. 2014. “Successful Supercooled Liver Storage for 4 Days.” Nature Medicine, 20(7): 790-93.
- “NIH-funded researchers extend liver preservation for transplantation.”
https://www.nibib.nih.gov/news-events/newsroom/nih-funded-researchers-extend-liver-preservation-transplantation
To Learn More:
- Center for Engineering in Medicine at Massachusetts General Hospital.
http://www.massgeneral.org/cem/
- Department of Health and Human Services Division of Organ Transplantation.
https://organdonor.gov/about-dot.html
- Organ Preservation Alliance.
https://www.organpreservationalliance.org/
- American Society of Transplantation.
https://www.myast.org/meetings/summit-organ-banking-through-converging-technologies
- MedLine Plus.
https://medlineplus.gov/organtransplantation.html
- World Health Organization.
http://www.who.int/transplantation/organ/en/
- Wait Not in Vain.
https://www.economist.com/news/science-and-technology/21690025-after-decades-piecemeal-progress-science-cryogenically-storing-human
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com

