| |
When an accident occurs on the highway, there’s a roadblock and no cars can get through. When cholesterol clogs an artery, the same thing happens: no blood can flow past the clog. But what if you could create a detour around the roadblock? Researchers at Dartmouth Medical School think they just might have found a way?
Have you ever wondered why a cut bleeds? A wound causes bleeding because it has broken a blood vessel part of the intricate network of veins, arteries, and capillaries that move blood around the body and provide the entire body with nutrients. Your cut heals as your body repairs the blood vessel. This biological process of repairing damaged blood vessels and creating new ones is known as angiogenesis .
Of course, in some cases of serious damage, you might have to have surgery to repair a blood vessel. This month, however, we are talking about nature’s process.
Throughout the entire body, blood vessels are made of a single layer of endothelial cells. When an organ such as skin, the liver, or the stomach, is not receiving enough oxygen, it releases chemicals called angiogenic growth factors into the blood stream. The endothelial cells recognize the growth factors; they are stimulated to multiply and form new blood vessels.
The new vessels, in turn, bring more blood to the tissue.
Angiogenesis occurs many times throughout life. For embryos in the womb, angiogenesis creates the whole vascular system. The heart serves as the pump that provides the force to push the blood around the body to all of the organs. As the body continues to grow, new blood vessels are formed in order to reach the growing tissues. When you stop growing, most angiogenic processes cease as well. Although your body will continue to repair damaged blood vessels, like when you get a cut, it is no longer regularly stimulated to grow brand new ones.
 Dr. Michael Simons and a gregarious team of researchers at Dartmouth Medical School in New Hampshire wanted to find the chemical responsible for responding to angiogenic growth factors in endothelial cells. Dr. Michael Simons and a gregarious team of researchers at Dartmouth Medical School in New Hampshire wanted to find the chemical responsible for responding to angiogenic growth factors in endothelial cells.
Their research focused on the expression of the chemical synectin in zebrafish embryos and mice. The team studied zebrafish embryos and mice that were specially bred without the gene for synectin: they are called knockdown zebrafish embryos and knockout mice, because one of their genes has been removed. The researchers compared arterial growth in the knockdown embryos and the knockout mice with arterial growth of normal animals called controls. The arteries of knockdown zebrafish embryos and knockout mice did not grow as well as the arteries of control animals. This resulted in mice that were smaller than their normal counterparts and would not have survived outside the lab. The stunted arterial growth in zebrafish embryos was so severe that none of the embryos survived until birth.
 The researchers found that synectin only affects arteries, not veins. This is the first time any distinction has been made between the two types of blood vessels. A chemical that acts similarly in veins has not yet been discovered. And why there is a difference between arteries and veins: “We would like to know, too,” says Dr. Simons. The researchers found that synectin only affects arteries, not veins. This is the first time any distinction has been made between the two types of blood vessels. A chemical that acts similarly in veins has not yet been discovered. And why there is a difference between arteries and veins: “We would like to know, too,” says Dr. Simons.
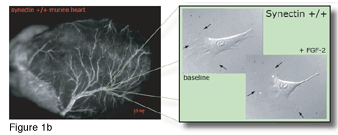 Figure 1a shows the stunted development of the heart of a synectin-knockout mouse compared to the development of the heart of a control mouse (Figure 1b). The heart of the synectin-knockout mouse is smaller and has a significantly reduced number of arteries that are smaller than the control mouse. Figure 1a shows the stunted development of the heart of a synectin-knockout mouse compared to the development of the heart of a control mouse (Figure 1b). The heart of the synectin-knockout mouse is smaller and has a significantly reduced number of arteries that are smaller than the control mouse.
Dr. Simons used zebrafish in addition to mice because these animals were the first organisms to develop a vascular system. “The fact that the zebrafish and the mice responded the same way to the under-expression of synectin shows how fundamental the process of angiogenesis is,” says Dr. Simons.
From their findings, the researchers concluded that synectin is crucial the angiogenic process in zebrafish as well as mice. Since the experiment showed a decrease in the animal’s size along with a decrease in arterial vasculature, the researchers now believe that the size of the vascular system is an important factor in determining the size of an animal. For example, if researchers could find a way to create an extremely large vascular system in mice, they could potentially create a mouse the size of an elephant!
A more beneficial application of these findings is to develop gene therapy for human coronary heart disease. Heart disease occurs when cholesterol clogs an artery, not allowing for vital oxygen and nutrients to get to other organs. It is the number one killer in the western world. Currently the only treatments for heart disease are cardiac bypass surgery (to replace the clogged vessel) or angioplasty (to unclog the vessel). These treatments involve major surgery and involve health risks and potential side effects.
Dr. Simons believes that the protein synectin in the endothelial cells regulates how the endothelial cells respond to growth factors sent by the oxygen-deprived organs. Having received the growth factors, synectin then stimulates the cells to reproduce and make new blood vessels. If he could find a way to over-express synectin, it could be possible to stimulate the body to grow new blood vessels around the clog.
He hopes that in the future gene therapy will be another, less invasive, option. For example, if researchers could find a way to over-express synectin, they could stimulate the body to create its own bypass around the obstruction. “This type of therapy may take years to develop,” says Dr. Simons. “Right now we are trying to understand how the system works.”
| Dr. Michael Simons is a Professor of Medicine, Cardiology Section Chief, and the director of the Angiogenesis Research Institute at Dartmouth Medical School in New Hampshire. His research focuses on the interactions of angiogenic growth factors with endothelial cells and the role of synectin in angiogenesis. He became interested in science in college where he wanted to explore how things work. He chose cardiology because of its importance to the medical field and the close link between research and clinical practice. |
 |

Dr. Michael Simons |
To Learn More:
- Chittenden T, Claes F, Lanahan A, Autiero M, Palac R, Tkachenko E, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh J, Horowitz A, Mullican-Kehoe M, Moodle K, Zhuang Z, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Cell 10:783-795, 2006.
- Carter, L. Sanguine Manner. Dartmouth Medicine Magazine, Summer 2006.
About
Angiogenesis:
About
Gene Therapy:
Written by
Rebecca Kranz with
Andrea R. Gwosdow, PhD
Gwosdow Associates
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important.
|
|
 |

| Click here for a time-lapse animation or arterial formation. |
| |
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts Society for Medical Research.
|
|

