|
It’s happened to all of us at some time or another: the camera battery dies and you can’t snap that last good picture, the watch battery dies and you’re left timeless for a while.
Our world runs on batteries that run out of fuel, sometimes at just the wrong moment. In some cases, battery death can mean actual life or death for the user. This is why medical devices such as
pacemakers,
need to be monitored extremely carefully and replaced long before their batteries fail. Such monitoring can be costly, not to mention inconvenient for the patient, and battery failure remains a constant concern.
If pacemakers and other battery-powered medical devices could fuel themselves it would greatly reduce the costs and risks of using the device. This is the ultimate goal of the research led by Dr. Evgeny Katz in the Laboratory of Bioelectronics and Bionanotechnology at Clarkson University in New York. Dr. Katz and his team have recently shown how snails, lobsters, and clams can power miniature batteries implanted inside them. These little batteries are called
biofuel cells.
To understand Dr. Katz’s research, let’s first take a look at the workings of your generic AA or AAA battery. Batteries work by extracting electrical energy from chemicals. Each battery has a positive terminal attached to the
cathode
and a negative terminal attached to the
anode.
The cathode and anode—together known as electrodes —are where the chemical reactions that power the battery take place. Although they don’t touch directly, a fluid known as an
electrolyte
allows electric charge to flow between the two poles.
When the battery is attached to a light bulb or watch, chemical reactions occur at both the anode and cathode to produce electricity inside the battery. These two reactions occur almost simultaneously and will continue to occur until one or both of the
electrodes
run out of material for the chemical reactions and the battery “dies”.
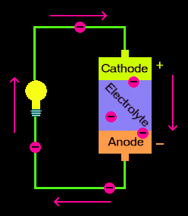
Battery schematic
Biofuel cells may look different, but they work in the same way.
Glucose
and oxygen provide the material for the chemical reactions that power Dr. Katz’s fuel cells. The anode and cathode reactions need to happen very quickly, so the biofuel cells contain two
enzymes
, an anode side to catalyze the oxidation of glucose and a cathode side to catalyze the reduction of oxygen.
The enzyme on the anode side is called pyrroloquinoline quinone-dependent glucose dehydrogenase (PQQ-GDH). The one on the cathode side is laccase. The enzymes are placed separately on a material composed of compressed carbon nanotubes called buckypaper. In order to create a living battery, Dr. Katz and his team implanted both the anode and the cathode into the animal. The glucose and oxygen within the organism use the organism’s blood as the electrolyte to create electricity. With the snails, the presence of glucose and oxygen results in electric currents.
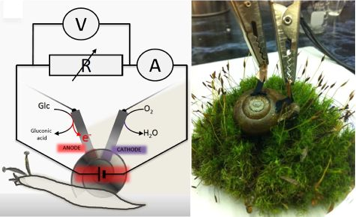
Biofuel cell-implanted snail
Dr. Katz’s ultimate goal is to create a device that could be powered by a person’s glucose to power implanted biomedical devices. Such a system is still a long way off. Other potential applications include systems for environmental monitoring, pollution control, and the detection of explosive and chemical weapons.
Dr. Evgeny Katz is the Milton Kirker Chaired Professor of Colloid Science at Clarkson University. He has recently been listed as one of the world’s most cited chemists. Dr. Katz first became interested in science through reading science fiction, and he now enjoys guiding his students to successful careers in science and chemistry. Dr. Katz enjoys traveling in his spare time.
To Learn More:
- What is Bionanotechnology? www.youtube.com/watch?v=ITtGJUGXFKc
- Institute for Bionanotechnology in Medicine www.ibnam.northwestern.edu
For More Information:
- Halamkova, L. et al. 2012. “Implanted Biofuel Cell Operating in a Living Snail.” Journal of the American Chemical Society, 134: 5040-5043.
- Szczupak, A. et al. 2012. “Living battery—biofuel cells operating in vivo in clams.” Energy and Environmental Science5: 8891-8895.
Podcast:
This is an informative podcast (and transcript) from the American Chemical Society.
Click here.
Videos:
Here’s a perky video showing how dry cell batteries work: www.youtube.com/watch?v=KkRwuM4S8BQ
Written by Rebecca Kranz with Andrea Gwosdow, PhD www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

