Highlights
Neurons use mitochondria to transport energy in two directions: from the cell body to the ends of the neuron and from the ends back to the cell body. Researchers found that when two neurons are connected via a synapse the amount of mitochondrial transport nearly doubles. This increase in bi-directional mitochondrial transport was regulated by the signaling pathway cyclic adenosine monophosphate (cAMP). Formation of the synapse irreversibly changed the pre-synaptic neuron, causing permanent changes in gene expression.
Did you know that there are around 200 different types of cells in your body? Each of these cells have specialized functions such as the electrical properties of the heart and brain cells or light-receiving properties of
photoreceptor cells
in the eye. One property that cells in the body share is that they need energy.
Energy in the cell is produced by an
organelle
called the
mitochondria.
Mitochondria process nutrients in the body into high-energy molecules used to power the cell. Depending on the cell type and location, a cell may contain between 1,000 and 2,000 mitochondria.
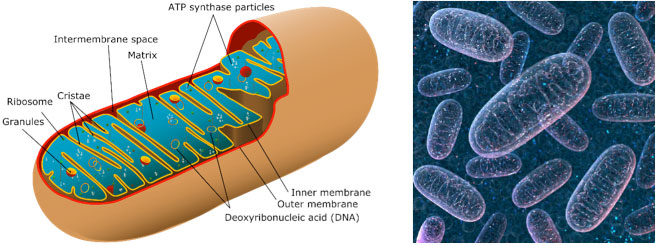
Figure 1. a) Picture of mitochondria with section cut open [Source:https://www.zmescience.com/other/science-abc/about-mitochondrial-dna-42423/ and b) 3-D image of mitochondria [Source: https://www.techexplorist.com/not-moms-genes-mitochondrial-dna-come-dad/18983/]
Mitochondria move within the cell in order to provide energy for different cellular functions. Some researchers hypothesize that problems with mitochondrial transport within cells of the nervous system might be an underlying factor in
neurodegenerative diseases
such as
Alzheimer’s disease,
Huntington’s disease,
and
Parkinson's disease.
This is the basis of new research by Dr. Sathya Puthanveettil and a team of researchers at Scripps Research Institute-Florida. To better understand their findings, let’s first explore how nerve cells function.
Nerve cells, known as
neurons,
are found throughout the nervous system including the brain, nerves, and spinal cord. Neurons are composed of a cell body, where the core functions of the cell take place, as well as
dendrites,
which are spine-like projections from the cell body that receive and transmit electrical signals. Dendrites are the shorter projections that receive electrical signals from other neurons. The typical neuron has multiple dendrites that extend into the network of neurons to receive electrical signals. Once a signal has been received, it is transmitted to the next neuron across a longer projection known as the
axon.
The typical neuron has only one axon.
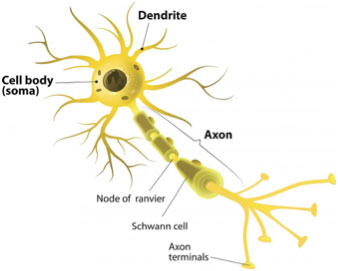
Figure 2. Picture of a typical neuron
[Source:https://www.medicalnewstoday.com/articles/320289.php]
Electrical signals are transmitted from one neuron to another using molecules known as
neurotransmitters.
These molecules are transmitted across the space between the axon of the first neuron and the dendrite of the second. This space is known as the
synapse.
The neuron transmitting the signal is called the pre-synaptic neuron and the neuron receiving the signal is known as the post-synaptic neuron.
The process of sending and receiving signals between neurons requires energy, so the majority of mitochondria in neurons are found close to the synapse, at the ends of the dendrites and axons. Since most mitochondria are produced near the cell body, they must be
transported to where they are needed. As the mitochondria age, are damaged, or can no longer perform their function, they must be transported back to the cell body to be removed.
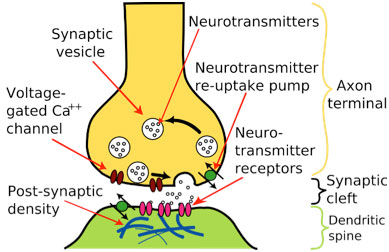
Figure 3. Picture of the end of the pre-synaptic neuron, synapse, and dendrites of the post-synaptic neuron
[Source:https://simple.wikipedia.org/wiki/Chemical_synapse]
“You can think of this mitochondrial transport system like parallel train tracks,” explained Dr. Sathya Puthanveettil. “Understanding how these train tracks function will increase our knowledge of memory and memory-related diseases.” These tracks are formed by the
cytoskeleton,
in what are known as
microtubules.
Proteins such as alpha- and beta-tubulin form the core of microtubule tracks.
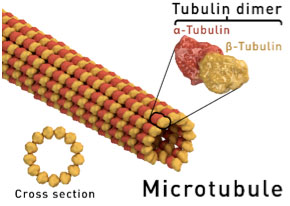
Figure 4. Picture of microtubule and tubulin dimers
[Source:https://en.wikipedia.org/wiki/Microtubule]
Along these train tracks, mitochondria are transported in both directions: from the cell body to the dendrites and axons, and from the axons and dendrites to the cell body. In order to better understand the role of mitochondrial transport in nerve cells, Dr. Puthanveettil and colleagues conducted experiments using the nerve cells of the sea slug, Aplysia californica.
Dr. Puthanveettil chose the sea slug because its neurons are relatively large in size and its nervous system is quite simple. In addition, processes like synapse formation and brain function are known to be conserved across species, meaning that they have not changed significantly over evolutionary time. Studying this simpler model can provide valuable information and insights into the brain function of humans and other animals.
The researchers began their experiments by looking at a single neuron in a petri dish. They used several measurements to specifically quantify mitochondrial transport, flux and velocity. Velocity refers to the speed of the mitochondria and flux refers to the number of mitochondria that pass a designated place on the axon in a given time period.
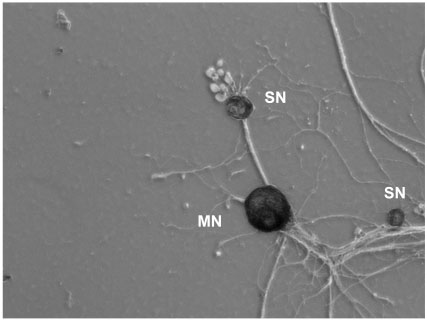
Figure 5. Aplysia californica sensory-motor neuron cultures. SN: Sensory neuron, MN: Motor neuron [Image credit: Kerriann Badal, Puthanveettil laboratory]
The researchers examined how different factors affect mitochondrial transport. For example, from previous research they knew that one of the critical components of transport in cells are microtubules. They are formed by long chains of tubulin, as needed by the cell (Figure 4).
In the experiments, the researchers exposed the neuron in the petri dish to the chemical nocodazole, which is known to interfere with the formation of microtubules. They observed that bi-directional mitochondrial flux decreased but velocity stayed the same. These findings show that microtubules are necessary for mitochondrial flux.
Next, the researchers compared mitochondrial transport in a single cell to mitochondrial transport of a
neural circuit
with two neurons. When two neurons were grown together, they formed a synapse within a few hours and were studied for an additional five to seven days. To confirm the presence of a synapse, the researchers used electrodes to measure electricity. Analysis of bi-directional mitochondrial transport of the two-neuron system showed a significant increase in flux without changes in velocity. This suggests that the formation of a synapse results in an increased number of mitochondria moving throughout the cell.
These findings left the researchers with a lingering question: what caused the increase in mitochondria in the two-neuron system? The researchers had two hypotheses: the increase resulted from the formation of the synapse, or from the two neurons constantly talking to each other.
To find the answer, the researchers grew another two-neuron system where the two neurons could not communicate across a synapse. Under this condition, there was no change in flux or velocity between the single neuron and the two-neuron system. The researchers therefore concluded that synapse formation causes the observed increase in bi-directional mitochondrial transport.
To better understand what happens to mitochondrial transport as the synapse forms, the researchers conducted time-lapse experiments. They measured the flux and velocity of mitochondria every six hours in the two-neuron system. After 24 hours, the researchers observed an increase in flux to and from the cell body (bi-directional) with no change in velocity.
To determine whether the observed transport patterns were specific to mitochondria or more broadly associated with other organelles, the researchers conducted similar time-lapse experiments with other organelles. They found that each organelle is regulated differently, so the mechanisms they studied for mitochondria affected only mitochondria.
To understand how mitochondrial transport is regulated, the researchers investigated different signaling pathways. They exposed the two-neuron circuit to compounds known to activate or inhibit common signaling pathways and observed the results. The findings from these experiments indicate the cyclic adenosine monophosphate (cAMP) pathway was important in the regulation of mitochondrial transport.
Finally, the researchers wanted to understand how the post-synaptic neuron affected the genetic expression of the pre-synaptic neuron. To do this, they analyzed the genetic profiles of the cell bodies of the pre-synaptic neurons before and after the formation of the synapse. They found that approximately 4,000 genes were activated after the formation of the synapse and continued to be activated for the duration of the experiments. About half of the activated genes were related to increased metabolism, indicating the need for more energy.
In summary, the researchers found connection to a post-synaptic neuron nearly doubled the amount of mitochondrial transport that occurred in the pre-synaptic neuron. This increase in bi-directional mitochondrial transport was regulated by the signaling pathway cAMP, which is also involved in many other cellular functions including cell growth and protein expression. In addition, the increase in mitochondrial transport persisted for the duration of the experiment and caused changes in gene expression for 4,000 genes, which appear to be permanent.
“Our most important finding to date is that the formation of the synapse irreversibly changes the pre-synaptic neuron,” commented Dr. Puthanveettil. The researchers hypothesize that increased mitochondria are needed for both the initial formation of the synapse and then for continued maintenance of the synapse.
In upcoming experiments, Dr. Puthanveettil and his team plan to explore how the regulation of mitochondrial transport is involved in memory.
Dr. Sathya Puthanveettil is Associate Professor of Neuroscience at Scripps Research Institute. His research focuses on the molecular basis of long-term memory. When not in the laboratory, Dr. Puthanveettil enjoys walking, playing tennis, and listening to classical music.
For More Information:
- Badal, K. et al. 2019. “Synapse formation activates a transcriptional program for persistent enhancement in the bi-directional transport of mitochondria.” Cell Reports, 26:507-17.
- Puthanveettil, S. et al. 2008. “A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex.” Cell, 135: 960-73.
- Swarnkar, S. et al. 2018. “Kinesin family of proteins Kif11 and Kif21B act as inhibitory constraints of excitatory synaptic transmission through distinct mechanisms.” Scientific Reports, 8.
To Learn More:
- Sathya Puthanveettil Laboratory. https://www.scripps.edu/puthanveettil/
- Society for Neuroscience. https://www.sfn.org/
- Neuroscience@NIH. https://neuroscience.nih.gov/
- Brain Basics: The Life and Death of a Neuron. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Life-and-Death-Neuron
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

