| |
Cancer cells and healthy cells differ in important ways. Cancer cells are characterized by uncontrolled growth; they do not respond to signals from other cells and they do not necessarily stay in their place of origin. This is what makes cancer so difficult to treat. Thousands of researchers across the globe are working to identify differences on the molecular and genetic level to effectively deliver targeted therapies and cure cancers.
Over the years we have highlighted some of these researchers including
Drs. Donald Ingber and Ofra Benny in 2008,
Dr. Jian-Wei Gu in 2013,
and
Dr. Jack Hoopes in 2015.
They and their colleagues are working to identify and exploit differences between cancerous and healthy tissue to improve treatment options and prevent future illness. Yet, despite the numerous differences between cancerous and healthy cells, it can be difficult, nearly impossible, to actually distinguish living healthy tissue from cancerous tissue.
"Cancerous tissue that eerily lights up a brain scan can appear no different from healthy tissue when we actually look at the patient's brain. We need tools to guide the surgeon in identifying cancerous tissue in the operating room. That's our goal."
"Cancerous tissue that eerily lights up a brain scan can appear no different from healthy tissue when we actually look at the patient's brain," says Dr. Jim Olson, a pediatric neuro-oncologist at the Fred Hutchinson Cancer Research Institute in Seattle, Washington. Dr. Olson's patients are children with brain tumors and their families. More traditional treatments such as radiation and chemotherapy may help shrink the tumor and prevent further cancerous growth, but brain surgery is often the first and only line of defense. "We need tools to guide the surgeon in identifying cancerous tissue in the operating room," explains Dr. Olson. "That's our goal."
When Dr. Olson first voiced the idea to light up cancer tissue, he was still a graduate student at the University of Michigan. As part of his graduate work, Dr. Olson helped develop a molecule that would selectively bind to cancerous tissue and not healthy tissue. He worked on a team that targeted trace amounts of radiation to brain tumors for diagnosis by
positron emission tomography (PET scanning).
"While defending my PhD research, a professor asked me what I would do next. Without really thinking about it, I said that if we could deliver radioactivity to cancer cells, why not make them light up? I would have never guessed at the time that would become my life's work."
In subsequent research positions, Dr. Olson became increasingly interested in the brain, eventually specializing in brain cancers in children. The idea to light up cancerous tissue was always there, and it became more urgent as so many of his patients underwent brain surgery. Brain surgeons count in millimeters, and even the removal of a small portion of healthy tissue can have devastating consequences for the patient. On the other hand, the consequences of leaving a small amount of cancerous tissue behind can be equally disastrous. The need for surgeons to be able to distinguish cancerous tissue from healthy tissue in the brains of their patients was abundantly clear.
Brain surgeons count in millimeters, and even the removal of a small portion of healthy tissue can have devastating consequences for the patient. On the other hand, the consequences of leaving a small amount of cancerous tissue behind can be equally disastrous.
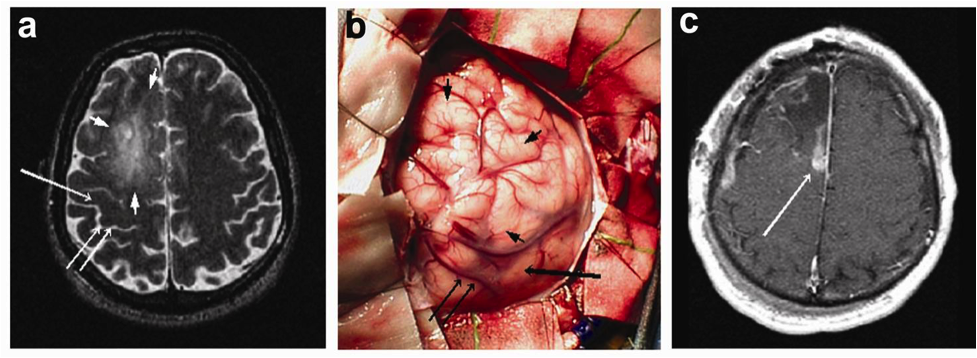
Picture of tumor locations in brain scans are highlighted by arrows in images A and C. Image B illustrates these same locations from images A and C during open surgery, exposing the difficulties brain surgeons encounter during tumor resection.
The basic goal was straightforward: to identify a molecule that would bind selectively to cancerous tissue and attach a fluorescent protein to it. Dr. Olson had already been exposed to some of this work during his graduate studies. The brain, though, poses an extra difficulty, because of the
blood-brain barrier.
The blood-brain barrier is a membrane that selectively allows only certain molecules into the brain. This is a protective mechanism to prevent potentially harmful molecules from nearing the brain. It serves us well. On the other hand, the blood-brain barrier also makes drug delivery complicated, because researchers must develop molecules capable of bypassing a barrier whose purpose is to keep them out.
Dr. Olson was aided in the initial stages of this project by a neurosurgery resident who wanted to identify differences between healthy brain tissue and brain tumor tissue. "There were lots of researchers doing this at the time," recalls Dr. Olson. So for six weeks this student spent at least eight hours per day on the computer, attempting to identify possible compounds that would bypass the blood-brain barrier. At the end of each day the student would present candidates to Dr. Olson. "And I proceeded to reject each idea for one reason or another," laughs Dr. Olson.
Until one day the student found a study done by a group of researchers from Alabama who were studying a molecule from the
Deathstalker Scorpion, Leiurus quinquestriatus
found in the desert regions of northern Africa and the Middle East.
"I thought, if anything knows how to bypass the blood-brain barrier, it's a scorpion," recalls Dr. Olson. Scorpions kill their prey by using
neurotoxins
- poisons that function by getting through the blood-brain barrier and entering the brain. In particular, this molecule is called chlorotoxin because it works by blocking chloride channels in the brain that are vital for normal function.
Chemical structure of chlorotoxin [from http://chemistry.umeche.maine.edu/CHY431/OddEnd2.html]
Having identified a potential candidate for further study, Dr. Olson and his team set out to test the scorpion-inspired molecule in mice.
Having identified a potential candidate for further study, Dr. Olson and his team set out to test the scorpion-inspired molecule in mice. They attached a fluorescent protein to the chlorotoxin and injected it into mouse models of brain cancer. And it worked. The cancerous tissue - but not the normal brain - became visible.
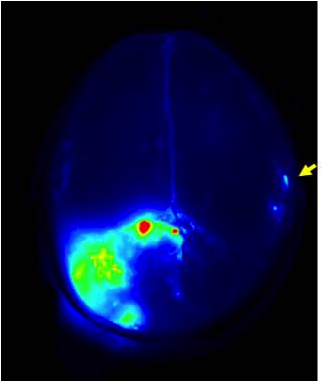
Fluorescent brain scan showing the fluorescence provided by Tumor Paint, with the cancerous cells highlighted. The blue-green glow in the right quadrant indicates additional cancerous tissue, demonstrating the high sensitivity of tumor paint (Blaze Bioscience Inc.)
The results were encouraging, and Dr. Olson and his team set out to replicate their results. Brain cancers account for only about 4% of all cancers, though, and Dr. Olson wondered whether the molecule his team had created could work for other types of cancers as well. He reached out to colleagues working on prostate, breast and skin cancer, achieving similarly positive results.
The findings were promising. But Dr. Olson knew that many treatments work well in mice but not in humans. As an intermediate step, Dr. Olson initiated a collaboration with the veterinary team at Washington State University to put out an open call for dogs with cancer. These were dogs whose owners had brought them to the veterinary clinic for surgery. The owners agreed to let the veterinary surgeons inject their pets with the fluorescent molecules created in Dr. Olson's laboratory. "The tumors lit up beautifully," Dr. Olson recalls. "It dramatically changed the surgical experience."
With success in dog patients, the molecule, now known as BLZ-100 tumor paint, has entered into human clinical trials conducted by Blaze Bioscience, a company that spun out of the Fred Hutchinson Cancer Center. The first clinical trials were conducted with patients with skin cancer to determine whether the tumor paint caused any adverse effects in humans. The molecule was found to be clinically safe, and tumor paint has now been tried in seven patients with brain cancer. In all cases the tumor paint has highlighted tumor tissue but not healthy tissue.
After nearly a decade of research, tumor paint is making its way into the operating room, providing surgeons with a vital tool to improve patient outcomes. "In ten years we will look back at a time when we conducted surgery without tumor paint as barbaric," comments Dr. Olson.
As tumor paint moved into clinical trials, Dr. Olson began to think about other applications for this type of work. He coined the word "optide," meaning "optimized peptide" to describe natural proteins (peptides) that have been modified for use in treatment. "The possibilities for work with optides are limited only by our imagination," emphasizes Dr. Olson.
His team includes advisers from Microsoft, Google, and other software companies, along with biologists, chemists, and engineers.
One of several projects underway in Dr. Olson's laboratory focuses on
Alzheimer's disease,
a progressive neurological disorder caused by a build-up of proteins in the brain that stick together and interfere with normal functioning. The researchers are developing an optide that would bind to proteins and prevent them from sticking together.
Dr. Olson emphasizes that this research is only made possible through a strong commitment to collaborative and interdisciplinary work. His team includes advisers from Microsoft, Google, and other software companies, along with biologists, chemists, and engineers. Team members come from the world of academia and business, clinicians and researchers alike. "The coolest things in science happen when you take people from every background and put them together on a really hard problem," comments Dr. Olson. Tumor paint, with the potential to help over two million patients in the U.S. and Europe alone, is one example. As Dr. Olson concludes, "I want kids to know that no matter what field they choose, no matter where their interests lie, everyone has something to contribute to science."
Dr. Jim Olson is a Principal Investigator at the Fred Hutchinson Cancer Research Center in Seattle, Washington and Assistant Professor of Pediatric Hematology/Oncology at the University of Washington School of Medicine. Dr. Olson is a researcher and clinician who also sees patients on a regular basis. He has developed tumor paint, the first of a class of molecules he has called "optides" or "optimized peptides" with wide applications in cancer research and beyond. When not in the laboratory, clinic or classroom, Dr. Olson enjoys outdoor activities and spending time with his family.
For More Information:
- Glowing "Tumor Paint" Shows Surgeons Where to Cut.: Scorpion Venom Lights the Way. April 8, 2015. Available at http://www.popsci.com/paint-lights-tumor-cells-brain.
- Akcan M, Stroud MR, Hansen SJ et al. Chemical Re-engineering of Chlorotoxin Improves Bioconjugation Properties for Tumor Imaging and Targeted Therapy J Med Chem 2011, 54 (3), pp 782-787
- Veiseh M, Gabikian P, Bahrami SB et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res 2007;67:6882-6888.
To Learn More:
Tumor Paint and Optides
- Olson Laboratory. http://research.fhcrc.org/olson/en.html
- Project Violet. http://libguides.fhcrc.org/PVex
- Blaze Bioscience. http://www.blazebioscience.com/
Brain Cancers
- National Cancer Institute. http://www.cancer.gov/types/brain
- Cancer Treatment Centers of America. http://www.cancercenter.com/brain-cancer/learning/
- American Brain Tumor Association. http://www.abta.org/brain-tumor-information/
- National Brain Tumor Society. http://braintumor.org/brain-tumor-information/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

First row (left to right): Shanon Turnbaugh, Michelle Sangar, Mi-Youn Brusniak, Kristina Pilat, Emily Girard, Jane Carter, Dina Alcorn
Second row (l to r): Alex Watson, Mesfin Gewe, Jim Olson, William Johnsen, Andrew Mhyre, Midori Clarke, Michelle Sangar (in front of Midori), Ashok Bandaranayake
Third Row (l to r): Andy Strand (with sunglasses), Theo Sottero, Pete Rupert, Madison Wise, Colin Correnti, Roland Strong, Jake Herman
Back row (l to r): Willem de van der Schueren, Ryan Steele, Zach Crook, Eric Finn, Julian Simon, Connor Wojciak, Eric Finn, Skyler Burke, Edward Kraft
Media links

TED Talk on Tumor Paint

WBUR story
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

