| |
Highlights
Type 1 diabetes is thought to be an autoimmune disorder that destroys beta cells in the pancreas. As a result, the body cannot regulate the level of sugar (glucose) in the blood through the production of the hormone insulin. These researchers developed a way to stimulate stem cells to differentiate into pancreatic islet cells in the laboratory. These islet cells were transplanted into animals and found to regulate glucose and insulin levels in the body. The results from the Phase 1 clinical trial indicate that the first patient who received these transplanted islet cells was effectively cured of type 1 diabetes. Clinical trials are currently underway to study this potential new treatment.
According to the United States Centers for Disease Control and Prevention, approximately 37 million Americans have been diagnosed with
diabetes,
including 283,000 young adults under 20 years of age. Diabetes is a chronic medical condition characterized by changes in the body’s ability to utilize sugar (glucose). Sugar levels in the blood are regulated by the
pancreas,
which sits just below the stomach, between the gall bladder and the small intestine.
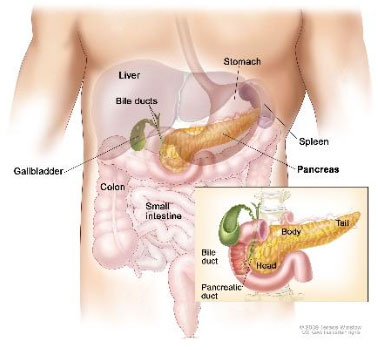
Figure 1. Pancreas in relation to other organs in the body
[Source: https://nci-media.cancer.gov/pdq/media/images/636528.jpg]
The area of the pancreas that regulates blood sugar levels is known as the Islets of Langerhans or
pancreatic islets.
The pancreatic islets contain two main types of hormone-producing cells that regulate blood sugar:
alpha
and
beta cells.
Alpha cells detect when blood sugar gets too low. When the alpha cells detect low blood sugar, they release the hormone
glucagon,
which stimulates the liver to release sugar into the blood stream. Alpha cells make up about 20% of islet cells.
Beta cells detect when blood sugar gets too high. When beta cells detect high blood sugar, they release the hormone
insulin,
which stimulates cells to absorb glucose from the blood. Beta cells make up the majority of islet cells, about 70%. Together, alpha and beta cells along with other cells found in the pancreatic islets are known as islet cells.
In people with diabetes, pancreatic islet cells are no longer able to properly regulate blood sugar. There are two main types of diabetes, known as type 1 and type 2. In type 2 diabetes, cells no longer respond properly to insulin, impacting the hormone’s ability to regulate blood sugar. Around 90-95% of people with diabetes have
type 2 diabetes,
which has been associated with
obesity
and excess sugar consumption. Anyone at any age can be diagnosed with type 2 diabetes.
Type 1 diabetes
is an autoimmune disease, a class of diseases that occur when the immune system mistakenly attacks healthy tissue. In the case of type 1 diabetes, the immune system destroys the insulin-producing beta cells in the pancreas, leaving the body unable to regulate and utilize sugar.
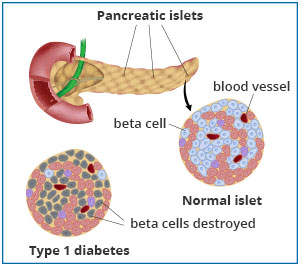
Figure 2. Comparison of healthy pancreatic tissue and pancreatic tissue affected by type 1 diabetes
[Source: https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatments/pancreatic-islet-transplantation]
Type 1 diabetes makes up about 5-10% of the total patient population with diabetes. It is most commonly diagnosed in children and young adults, although it is also possible to be diagnosed with type 1 diabetes as an adult.
Diabetes of all types is a chronic condition that can be treated and managed, but not cured. Management of diabetes includes lifestyle changes such as diet and exercise, oral and injected medications to help regulate blood sugar and stimulate the body to produce insulin, and injected insulin. Proper management of diabetes is important because, over time, uncontrolled blood sugars can cause a variety of complications including damage to the eyes, feet, kidneys, and nerves, as well as an increased risk of heart attack and stroke. People with diabetes must also monitor their blood sugars regularly and be aware of symptoms they might experience if their blood sugar drops too low or rises too high.
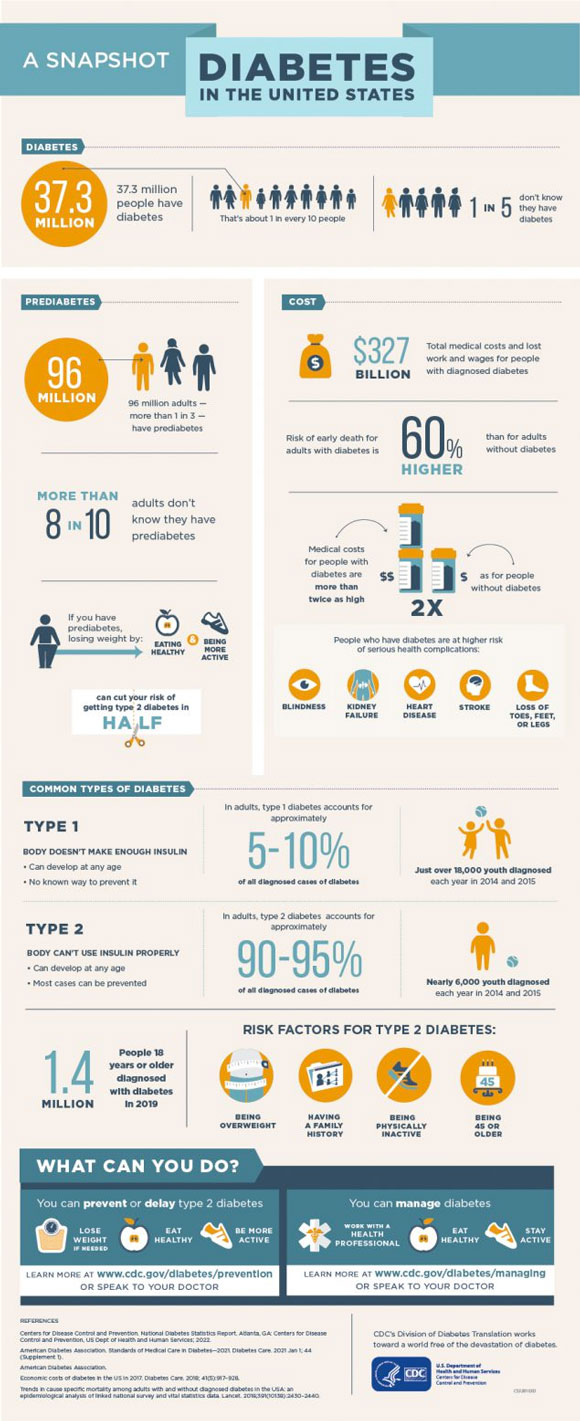
Figure 3. Infographic about diabetes from the Centers for Disease Control and Prevention
[Source: https://www.cdc.gov/diabetes/library/socialmedia/infographics/diabetes.html]
Disease management looks different for patients depending on whether they have type 1 or type 2 diabetes. When detected early, it is possible to reverse the development of type 2 diabetes by maintaining a healthy diet, exercising, and regularly checking in with your doctor. This is because people with type 2 diabetes have beta cells that are producing some insulin. Lifestyle modifications may stimulate the body to produce more insulin and increase the body’s ability to utilize insulin. Depending on the severity of the disease, management can include a variety of medications and, if these medications are insufficient, injecting insulin.
Figure 4. Vials of insulin, given by injection
[Source: https://en.wikipedia.org/wiki/Insulin (medication)]
In contrast, type 1 diabetes cannot be prevented or reversed, and management almost always includes injecting insulin, since the insulin-producing beta cells have been destroyed.
There is currently no cure for type 1 diabetes. However, new research by Dr. Douglas Melton, Xander University Professor of Stem Cell and Regenerative Biology and Co-Director of the Harvard Stem Cell Institute at Harvard University, has laid the foundation for a potential cure in the future.
From Stem Cells to Pancreatic Islet Cells
Dr. Douglas Melton began his scientific career studying animal development in frogs. When his first child was diagnosed with type 1 diabetes in 1991, he quickly shifted his research focus. “My new goal was to develop a cure for this disease,” recalled Dr. Melton. “I was looking, and am still looking, for a cure for my children.”
It was known at the time that type 1 diabetes was the result of the destruction of insulin-producing beta cells. “All I had to do,” remarked Dr. Melton, “was find a way to grow pancreatic islet cells, including beta cells, in the lab.” Dr. Melton believed this could work because research conducted 50 years prior had shown that transplanting pancreatic beta cells from a recently deceased cadaver to a patient with diabetes could cure the disease. However, there are not enough available organ transplants for this to be a feasible treatment option. Instead, Dr. Melton focused on creating laboratory-grown islet cells with the goal of transplanting them into patients with diabetes. The clusters of islet cells would include both alpha and beta cells.
In order to develop islet cells, Dr. Melton and his team began in the place that every cell in our body begins: with
stem cells.
Stem cells have the potential to develop into any kind of cell because they have not yet specialized or differentiated into a specific cell type. The kind of mature cell a stem cell will become depends on the environment the cell is exposed to, including a variety of different signaling molecules.
Dr. Melton and his team studied the process of pancreatic stem cell differentiation for years to come up with a combination of signaling molecules that would program a pancreatic stem cell into an islet cell in the laboratory. Signaling molecules include proteins produced by different cell types, such as growth factors, as well as components of vitamins, such as retinoic acid derived from Vitamin A.
The puzzle Dr. Melton and his team had to figure out was which molecules were needed at what point in development, in what amount, and for how much time. In 96-well plates, Dr. Melton combined pancreatic stem cells with the purified version of each signaling molecule to see what would happen.
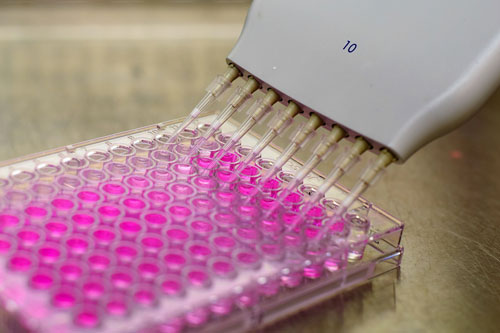
Figure 5. 96-well plate
[Source: https://commons.wikimedia.org/wiki/File:96_well_plate.jpg]
Dr. Melton and his team identified what happened to the stem cells by using antibodies related to specific gene products or by altering the stem cells so they would fluoresce under certain conditions. The researchers created over 150 different experimental combinations using over 70 different compounds shown to be involved in pancreatic biology. Of these 70 compounds, 11 were shown to significantly impact the development of a pancreatic stem cell into a pancreatic islet cell. The 150 combinations included different amounts of signaling molecules for different amounts of time at different points in the differentiation process.
In order to determine which of the different combinations of signaling molecules could best mimic human islet cells, Dr. Melton and his team tested the function of the laboratory-grown cells. For example, islet cells sense changes in blood glucose levels using calcium signaling. An increase in blood glucose levels leads to an influx of calcium ions into the cells. The researchers monitored calcium regulation in the laboratory-produced beta cells using
fluorescent microscopy
to make sure the cells could respond appropriately to calcium signaling.

Figure 6. Fluorescence microscope
[Source: https://en.wikipedia.org/wiki/Fluorescence_microscope]
In addition, the researchers analyzed protein and RNA expression of the cells to ensure they produced the compounds that islet cells produce.
When the researchers showed that a group of laboratory-produced islet cells functioned like human alpha and beta cells in the petri dish, the next step was to test the function of the cells in control mouse models. The first test was a glucose challenge, where the mice with transplanted islet cells were given glucose and the researchers observed whether and how much insulin the beta cells produced in response to this challenge. Dr. Melton and his team found that two weeks after transplanting the islet cells, the transplanted islet cells produced significant amounts of insulin in response to a glucose challenge.
Next, the researchers conducted similar experiments in a mouse model of diabetes. This mouse model has a
mutation
that leads to the failure of beta cells resulting in increasingly elevated blood glucose levels. When islet cells were transplanted into these mice, the transplanted islet cells were able to reduce the blood sugar levels back to normal as well as clear glucose from the blood after a glucose challenge. The cells were still producing insulin up to 18 weeks after transplantation.
Sometimes a combination that seemed to develop into functional islet cells would not produce insulin in the mouse model, or it would work in the control mouse model but not in the mouse model of diabetes. When this happened, the researchers had to go back to the first phase and try again with different variables.
Entering Clinical Trials
In 2014, over 20 years after his son’s initial diagnosis, Dr. Melton and his team finally developed a combination that stimulated significant insulin production in mouse models of diabetes. In order to move this research forward, Dr. Melton co-founded a company called Semma (now Vertex) to produce larger quantities of islet cells than was possible to grow in the laboratory. Semma, a combination of the names of Dr. Melton’s children with type 1 diabetes, Sam and Emma, was able to replicate similar results in rats, and then in larger animals including pigs. Each time, the laboratory-grown islet cells were shown to be safe and effective at stimulating insulin production.
Once Semma had shown the laboratory-produced islet cells were successful in larger animals, the company was acquired by a larger pharmaceutical company called Vertex that had the capacity to bring the potential treatment to human clinical trials.
As described in the February 2022 article “Clinical Trials Ensure COVID-19 Vaccines are Safe and Effective,” human clinical trials have three phases: Phase 1 tests for safety; Phase 2 tests for efficacy; and Phase 3 compares the new treatment to those already available.
In October 2021, Vertex announced that the first patient who had received the transplanted islet cells in the Phase I clinical trial had effectively been cured of type 1 diabetes. “This was astounding news,” commented Dr. Melton. It is important to keep in mind that this result was only the first patient in the first phase of a clinical trial that plans to enroll 17 more patients to focus on treatment safety. The first patient received half the intended treatment dose.
Future Work
While excited about these initial results, Dr. Melton also knows there is much more work to be done. The transplantation of islet cells requires ongoing suppression of the immune system in order to work. This is because the immune system was responsible for destroying the beta cells in the first place. If new beta cells were to appear without immunosuppression, the immune system would just destroy the transplanted cells too. As a result, in order for the current treatment to remain effective, patients must continue to take immunosuppressing medications for the rest of their lives.
Developing techniques to allow the transplanted islet cells to evade the immune system is the goal of Dr. Melton’s ongoing and future research. Dr. Melton is using signaling molecules that can successfully avoid the immune system as a guide. These include a protein used by cancer cells called protection against death ligand 1 (PDL1) and the cells found on the outside of a growing embryo called trophectoderm cells. “If we can add one of these cells to our islet cells,” Dr. Melton concluded, “we could make them look like something the immune system does not need to respond to. That’s our key to neutralizing the immune response.”
Dr. Douglas Melton is the Xander University Professor of Stem Cell and Regenerative Biology and Co-Director of the Harvard Stem Cell Institute at Harvard University and Investigator at Howard Hughes Medical Institute. His research has focused on developing a cure for type 1 diabetes for the past three decades. When not in the laboratory, Dr. Melton enjoys playing tennis and squash, riding his bike, walking his dogs, and spending time with his family.
To Learn More:
- Pagliuca, F. et al. 2014. “Generation of functional human pancreatic β cells in vitro.” Cell, 159(2): 428-39. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4617632/
- Kolata, G. 27 Nov 2021. “A Cure for Type 1 Diabetes? For One Man, It Seems to Have Worked.” New York Times. https://www.nytimes.com/2021/11/27/health/diabetes-cure-stem-cells.html
For More Information:
- Melton Lab. https://hscrb.harvard.edu/labs/melton-lab/ and https://www.themeltonlab.com/
- Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/type1.html
- American Diabetes Association. https://www.diabetes.org/diabetes/type-1
- Joslin Diabetes Center. https://www.joslin.org/patient-care/diabetes-education/diabetes-learning-center/what-type-1-diabetes
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. Douglas Melton

Left to right, back row:Jose Rivera Feliciano, Igor Nikolskiy 4th row: Jenny Kenty, Max Serrano, Tim Kunz, Stanley Dale, Nicolai Hathiramani, Alex Atkin 3rd row: Adrian Veres, Aubrey Faust, Elaine Robinson, Douglas Melton, Alana Van Dervort, Helen McGunnigle, Jenny Babon, Xavier Sanchez Moreno, Shlomi Brielle 2nd row: Elad Sintov, Sarah Atanasov, Xi Wang, Quan Zhau, Jingping Zhang Front row: Hongfei Li, Ramona Pop, Danny Bavli

Doug Melton: Renewable bodies

TedX

Curing Diabetes with Dr Douglas Melton
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

