| |
Recent estimates place the number of cells in the human body at over thirty trillion cells
(that’s 3 x 1013 or 30,000,000,000,000)
. Each of these cells contains tens of millions of molecules—
organelles
and proteins, among others—that collectively enable the cell to function. This means that just to continue existing, the human body depends on lots and lots of moving parts.
The key to our existence is communication. The brain must communicate with other parts of the body; the cells in one part of the brain must communicate with the cells in other parts of the brain; and the molecules within each cell must communicate with each other to make further communication possible. New research in the laboratory of Dr. David Sinclair at Harvard Medical School suggests that the breakdown in communication at the most basic, intracellular level in older bodies contributes significantly to the aging process. If this proves to be the case—and Dr. Sinclair thinks it will—it may be possible to alter when and how we age by restoring proper communication within our cells.
It may be possible to alter when and how we age by restoring proper communication within our cells.
Perhaps by now the story of aging is getting a bit old. Indeed, in the February 2014 article “How Old is Your Blood?” we also discussed aging, specifically the work of Dr. Morgan Carlson at the University of Connecticut: he studies the aging process of skeletal muscles in middle-aged mice.
Dr. Carlson’s research focused on particular factors in the blood that would communicate the need for new blood vessel growth to other cells.
Whereas Dr. Carlson investigates communication on the intercellular level—that is, between different cells, Dr. Sinclair focuses on intracellular communication—that is, how messages get transmitted within individual cells before they are even developed for transmission to neighboring cells.
Dr. Sinclair’s research begins with
mitochondria, self-contained organelles known as the “powerhouses” of the cell because they produce all the energy cells need to survive and communicate. Mitochondria are found in almost all living cells, and many degenerative diseases such as
Alzheimer’s disease
have been associated with mitochondrial degeneration. Most researchers hypothesized that this degeneration was due to genetic mutations in the mitochondrial DNA and, therefore, irreversible. Results from the work of Dr. Ana Gomes, post-doctoral fellow in Dr. Sinclair’s laboratory, however, indicated that something else might be going on.
“At the time,” Dr. Gomes explains, “we were working with mice that lacked a particular gene known to be associated with the aging process.” The researchers wanted to understand how the lack of this gene might accelerate the aging process in younger mice. The gene, known as SIRT-1, was one of a group of genes known as
sirtuins
that had been an important area of research in Dr. Sinclair’s laboratory for some time.
Dr. Gomes expected to find signs of premature aging in the mice lacking the SIRT-1 gene, including mitochondrial degeneration. But her results were surprising. There was, in fact, very little evidence of mitochondrial degeneration—most of the proteins produced by the mitochondria were normal. Only a small number of mitochondrial proteins were at significantly reduced levels. Further investigation revealed that all the proteins with reduced levels had one thing in common: they were encoded by genes found in the DNA of the mitochondria rather than the DNA of the nucleus of the cell.
Good communication between the nucleus of the cell and the mitochondria is essential in order for cells to function properly.
The next logical question, of course, was why? What Dr. Gomes and her colleagues found was quite remarkable. The mitochondrial degeneration the researchers found was not caused by genetic mutation, as had been expected. “We found instead a breakdown in communication,” Dr. Gomes explains. It turns out that good communication between the nucleus of the cell and the mitochondria is essential in order for cells to function properly. This intra-cellular communication involves a chain of cellular messages that begin with a protein known as
nicotinamide adenine dinucleotide
or NAD for short, and end with successful communication between the two organelles. In this case, SIRT-1 functions to make sure this process runs smoothly. SIRT-1 works by preventing the interference of another cellular protein known as
hypoxia inducible factor-1
, or HIF-1. For reasons the researchers do not yet know, as we age the amount of NAD decreases, resulting in an increase of HIF-1 that becomes too much for SIRT-1 to handle. As a result, communication between the mitochondria and the
nucleus
breaks down causing mitochondrial, and hence, cellular dysfunction. “This is the first time any such process has been described,” comments Dr. Gomes.
Having identified this new cellular pathway, Dr. Gomes conducted a series of experiments to test her hypothesis. In these experiments, Dr. Gomes examined the function of mitochondria in elderly mice before and after the administration of a compound called nicotinamide mononucleotide or NMN to restore NAD levels to their non-aging levels. She found that NMN, which the mice metabolize into extra NAD, reduced the effects of aging in the elderly mice in only a week’s time. The decreased oxygen levels and rates of respiration seen in older mitochondria were restored to levels seen in younger mice. “This was really an incredible result,” remarks Dr. Gomes.
In her current experiments, Dr. Gomes is using the same compound, NMN, in cancer cells to see if increased NAD levels can reverse cancerous growth in aging cells. She also plans to use this compound in combination with cancer treatments that already exist. “We are hoping for synergistic effects,” remarks Dr. Gomes. Of course, there is much more work to be done to better understand the new pathways for intracellular communication identified by Dr. Gomes in her aging work as well. “We have a long way to go,” Dr. Gomes comments. “But that’s what science is all about.”
Dr. Ana Gomes is a post-doctoral research fellow in the laboratory of Dr. David Sinclair at Harvard Medical School. She began her doctoral research in her native Portugal before coming to the United States to continue her studies. She completed her PhD in Dr. Sinclair's laboratory. When not in the laboratory, Dr. Gomes enjoys reading and going to concerts.
To Learn More:
- Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseduohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013;155:1624-1638.
- Bratic A, Larsson N-G. The role of mitochondria in aging. J Clin Invest 2013;123:951-957.
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends EndcrinolMetab 2010;21:134-141.
For More Information:
- What is mitochondria disease? www.umdf.org
- Cell structure: Mitochondria. www.biology4kids.com/files/cell_mito.html
- National Institute on Aging. www.nia.nih.gov
- Mitochondria www.nature.com/scitable/topicpage/mitochondria
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

The Sinclair Lab
(from left to right)
First row: David Sinclair
Second row: Susan DeStefano, Luis Rajman, Ricardo Godinez
Third row: Jun Li, Kristen Simmons, Neha Garg
Forth row: Ana Gomes, Michael Schultz, Yutaka Hasegawa
Fifth row: Motoshi Hayano, Eduardo Coronado, Yutaka Hasegawa
Sixth row: Jae-Hyun Yang

Dr. Ana Gomes
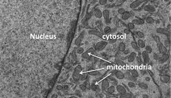
The mitochondria in a cell
Powering the Cell – Mitochondria
http://www.youtube.com/watch?v=RrS2uROUjK4
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

