Did you know that your brain contains approximately one billion
neurons?
Neurons are specialized cells found in the brain,
spinal cord,
and
nervous system
that are responsible for processing and transmitting information from the brain to the rest of the body and back to the nervous system. There are so many kinds of information that need to be processed. This information is related to movement, sensation, and thinking, among other functions. That is why there are about 10,000 distinct types of neurons that specialize in addressing different kinds of information.
In studying how the brain functions,
neuroscientists
have been able to describe many of the specific types of neurons in the brain. For example, in 2006, Dr. Joel Geerling and colleagues identified a group of neurons involved in regulating sodium-seeking behavior, known as sodium appetite. They identified the "sodium appetite" neurons by working backwards. From previous studies, the researchers knew that sodium deficiency caused the body to release a
hormone
known as
aldosterone.
Aldosterone increases the amount of sodium retained in the body. Importantly, aldosterone was also found to stimulate neurons located in the brain stem called "HSD2 neurons" because they express the
enzyme
hydroxysteroid dehydrogenase type 2 (HSD2).
When the amount of fluid in the body is too little, you become thirsty and water is retained, as shown in Figure 1.
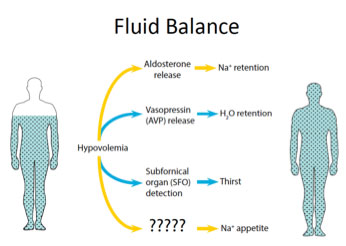
Figure 1. Fluid regulation in the body.
But what stimulates us to eat salty foods? This is the focus of the current research including post-doctoral fellow Dr. Jon Resch in the laboratory of Dr. Bradford Lowell at Beth Israel Deaconess Medical Center in Boston, MA. This laboratory has used the latest tools in neuroscience to figure out how the HSD2 neurons control sodium intake.
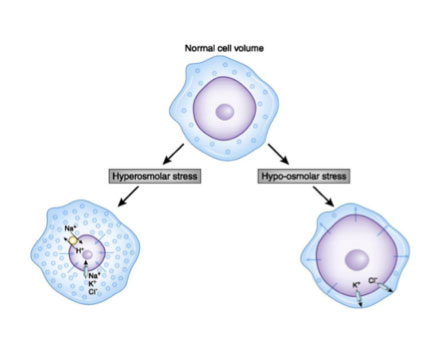
Figure 2. A normal cell compared to cells with too much sodium (hyperosmolar) and too little sodium (hypo-osmolar). [Adapted from Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852-62. doi: 10.2215/CJN.10741013. PubMed PMID: 25078421; PMCID: PMC4422250.]
Fluorescent Neurons
Dr. Resch and his colleagues were able to continue the work of Dr. Geerling because of significant advances in technology that have taken place over the last decade. Specifically, the researchers used a tool called the
cre-lox recombination system,
which uses
transgenic mice
to cause certain targeted neurons to become fluorescent so that researchers can visualize them.
Transgenic mouse models are mice that have one or more genes that are from a different animal. In the case of the cre-lox system, one set of transgenic mice, called the cre mice, contain the gene for the enzyme cre recombinase, originally found in bacteria, that recognizes and recombines specific DNA sequences known as lox sites. If a gene is located between two lox sites, the cre recombinase will delete the gene. The other set of transgenic mice, called the reporter mice, contain a fluorescent protein that is inactive because it is located between two lox sites. When the cre mice are crossed with the reporter mice, any cell that contains cre recombinase will remove the lox sites from the reporter mice and cause the cells to fluoresce.
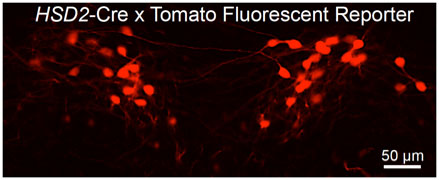
Figure 3. Picture of fluorescing cells.
With this new technology, Dr. Resch and colleagues measured changes in gene expression from HSD2 neurons based on sodium intake. To do this, they created cre-lox recombinant mice that would selectively fluoresce HSD2 neurons, allowing researchers to identify them when looking at the brain under the microscope. Dr. Resch and his team could then study individual neurons to see which genes were being expressed at that moment in time. They compared the genetic expression profile of the neurons from mice that had been deprived of sodium with control mice that had been given a normal diet. This allowed the researchers to understand the effect of sodium deficiency on the genetic expression of HSD2 neurons. From these experiments, the researchers identified two specific sodium channels that were increased in the sodium deficient mice compared to the control mice. This confirmed the researchers’ hypothesis that sodium deficiency caused additional sodium channels to open on HSD2 neurons.
Next, Dr. Resch and colleagues wanted to know whether HSD2 neurons were a necessary component of sodium regulation, meaning that the process would not occur in the absence of these neurons. To do this, they used an adaptation of the cre-lox model that selectively killed the HSD2 neurons. In the laboratory, these mice did not consume as much sodium as control mice, indicating the HSD2 neurons were essential for sodium appetite.
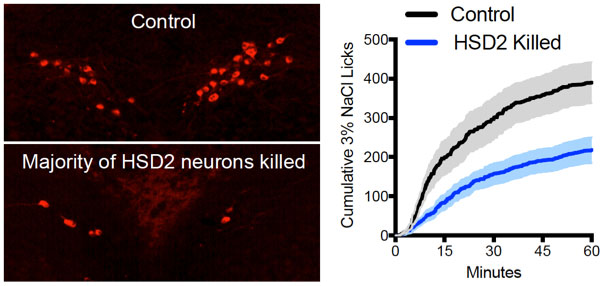
Figure 4. Selective killing of HSD2 neurons decreases sodium intake compared to controls.
Finally, the researchers wanted to determine whether HSD2 neurons were the only neurons that regulate sodium appetite, or whether there were other neurons involved as well. To do this, they further altered the cre-lox recombinase model so the HSD2 neurons were selectively activated. If only HSD2 neurons were involved in sodium appetite, the researchers expected selective activation of these neurons would lead to increased sodium consumption in mice. However, the researchers found that selective activation of the neurons had no effect on sodium regulation, indicating that there were other processes involved as well.
Neurons and Hormones
Dr. Resch and his team relied on previous knowledge of sodium regulation to hypothesize that the hormone
angiotensin
was also involved in sodium regulation. Angiotensin causes blood vessels to become narrower, increasing blood pressure and also sodium levels. The researchers hypothesized that HSD2 neuronal activation might cause the expected increase in sodium appetite in the presence of high angiotensin levels. In order to test this hypothesis, the researchers increased angiotensin levels in mice by depriving them of water. This time, activation of HSD2 neurons did cause the mice to consume more sodium.
To confirm that angiotensin was also necessary for sodium appetite, the researchers then conducted the same experiment, but this time the pathway for recognizing angiotensin levels was blocked. Without angiotensin
receptors
to notice the high angiotensin levels, the increased sodium appetite through activation of HSD2 neurons did not occur. As a result, the researchers concluded both angiotensin and HSD2 neuron activation were needed for sodium intake, and the process would not function correctly if either was missing.
Recall that in the original experiments conducted by Dr. Geerling and colleagues, the researchers identified the HSD2 neurons because they were sensitive to the hormone aldosterone. So what does angiotensin have to do with sodium intake? The researchers believe that both the neurons that respond to angiotensin and aldosterone (HSD2 neurons) transmit messages to the same part of the brain. Figure 5 shows how they would work together to control sodium appetite.
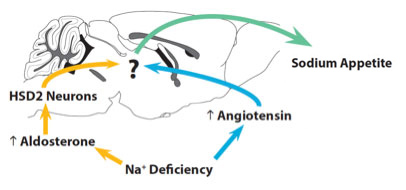
Figure 5. Regulation of sodium appetite by the brain.
In future experiments, the researchers hope to continue to explore the neuronal pathways regulating sodium appetite including angiotensin, aldosterone, and HSD2 neurons. “We hope this research leads to a better understanding of how sodium affects the cardiovascular system, and that knowledge will help save lives,” says Dr. Jon Resch.
Sodium is necessary to maintain blood pressure and can cause damage when consumed in excess. This is why people with high blood pressure or heart disease are told to limit their salt intake. This research could benefit people with high blood pressure and heart disease, as well as impact how we treat other disorders related to fluid imbalance or loss.
Dr. Jon Resch is a post-doctoral fellow in the laboratory of Dr. Bradford Lowell at Beth Israel Deaconess Medical Center in Boston, MA. When not in the laboratory, Dr. Resch enjoys playing ultimate frisbee and attending concerts and music festivals.
For More Information:
- Resch, J. et al. 2017. “Aldosterone-sensing neurons in the NTS exhibit state-dependent pacemaker activity and drive sodium appetite via synergy with angiotensin II signaling.” Neuron, 96: 190-206.
- Jarvie, BC and RD Palmiter. 2017. “HSD2 neurons in the hindbrain drive sodium appetite.” Nature Neuroscience, 20(2): 167-169.
- Geerling, J. et al. 2006. “Aldosterone target neurons in the nucleus tractus solitaries drive sodium appetite.” Journal of Neuroscience, 26(2): 411-417.
- Sakai, R. et al. 1986. “Salt appetite is suppressed by interference with angiotensin II and aldosterone.” American Journal of Physiology, 251: R762-8.
- Fluharty SJ and Epstein AN. 1983. “Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat. Behavioral Neuroscience, 97(5): 746-58.
To Learn More:
- Harvard Brain Science Initiative. https://brain.harvard.edu/people/bradford-lowell-md-phd
- #BreakUpWithSalt Sodium Reduction Initiative of the American Heart Association. https://sodiumbreakup.heart.org/
- Overview of Sodium’s Role in the Body. http://www.merckmanuals.com/home/hormonal-and-metabolic-disorders/electrolyte-balance/overview-of-sodium-s-role-in-the-body
- Sodium and Salt. http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/Sodium-and-Salt_UCM_303290_Article.jsp
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com

