Highlights
Cancer is often caused by genetic mutations that lead to abnormal cellular growth. One new treatment for cancer patients, called immunotherapy, focuses on boosting the immune system to fight cancer. One reason that immunotherapies may not work is that cancers use different mechanisms to adapt to and hide from the immune system.
These researchers compared the growth of tumor cells in mice with and without functioning immune systems to better understand the genes that contribute to a tumor’s ability to survive immune surveillance. The results of these experiments show at least 100 genes whose mutation leads to increased tumor growth only in the presence of the immune system. Further examination of these genes revealed that many are tumor suppressor genes, which are commonly mutated in patients with cancer.
In the future, these researchers plan to expand this study to include more of the genome of cancer cells. They also plan follow-up studies to better understand the function of specific genes with the goal of developing more targeted cancer treatments.
The
immune system
is made up of a network of cells, tissues, and organs that work together to neutralize any foreign agents that might enter the body. These foreign agents usually appear in the form of bacteria, virus, fungi, and other types of pathogens. However, sometimes the cells inside our body can also become agents of disease. This is what happens when someone has
cancer:
genetic changes in specific tissue types allow for abnormal cellular growth. These genetic changes can also signal to the body’s immune system that the cancer cells are abnormal.
There are thousands of researchers around the world who have spent their careers trying to develop more effective, targeted treatments for cancer patients. One advance in recent decades is
immunotherapy.
Immunotherapy is a class of cancer treatments that focuses on boosting the immune system to better recognize and defeat cancer cells. However, immunotherapy only works in a subset of cancer types. This is because some types of cancer have adapted ways to evade the immune system, making immunotherapy less effective.
In order for cancer immunotherapies to become more effective, researchers need to better understand the mechanisms used by cancer cells to hide from the immune system. New research by Dr. Timothy Martin and colleagues in the laboratory of Dr. Stephen Elledge at Harvard Medical School has identified over 100 genes that allow cancer cells to hide from the immune system. By better understanding these genes and how they function in different cancer types, this research lays the groundwork for more targeted forms of immunotherapy that can more successfully treat a variety of cancers.
Cancer Genetics
Much of the research in Dr. Elledge’s laboratory focuses on cancer genetics. Cancers arise out of genetic mutations in previously healthy cells, leading to abnormal cellular growth. In the field of cancer genetics, scientists study the genetic
mutations
that allow cancer to develop. Dr. Martin specializes in screening techniques to identify how these genetic mutations promote the disease-causing properties of cancer cells. He was approached by colleagues Dr. Danielle Cook and Dr. Kevin Haigis, who were interested in studying how tumors develop in
animal models
with and without functioning immune systems. Together they designed an experiment to try to identify the genes involved in allowing cancer cells to evade the immune system.
This new research was made possible by recent innovations in technologies that can alter genetic information in cells, namely
CRISPR.
CRISPR stands for clustered regularly interspaced short palindromic repeats. Using a specific protein that can cut DNA, called CRISPR-associated protein 9 or
Cas9,
CRISPR allows for the precise mutation of a single gene.
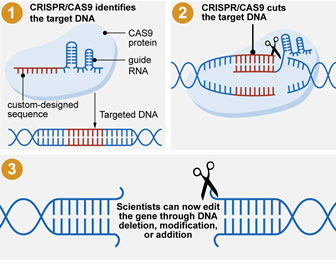
Figure 1. Infographic of how CRISPR and Cas9 work together to precisely edit genetic information
[Source: Government Accountability Office]
Dr. Martin and colleagues used CRISPR and Cas9 to create a library of tumor cells where each entry in the library was missing a single gene. For these experiments, the researchers used the 4T1 breast tumor cell line that is commonly used in cancer genetics research. Since the entire genetic code of the cells, also called the
genome,
had already been identified, the researchers could systematically delete a single gene from the cells using CRISPR. Dr. Martin and his team have used this technology to create a library of over 7,500 cells with a distinct gene missing.
The Role of the Immune System in Cancer
In order to determine how the deletion of each gene affected the growth and development of breast tumor cells, Dr. Martin and colleagues grew each cell entry from the library in petri dishes in the laboratory.
Figure 2. Cells growing in petri dishes
[Source: Dr. Martin]
Once the population of cells had doubled ten times, the researchers harvested the accumulation of cells that resulted and compared the abundance of CRISPR-mutated genes. In particular, Dr. Martin and his team were interested in the mutated genes that increased or decreased in abundance due to the CRISPR targeting.
Figure 3. Accumulation of cells in the petri dish
[Source: Dr. Martin]
The results of these initial experiments showed which genes were essential for the growth of breast cancer cells. To understand which genes were specifically involved in allowing cancer cells to evade the immune system, Dr. Martin and his team conducted a related series of experiments in mouse models. The researchers introduced cells from each of the entries in the cell library into two mouse models: a control mouse with an intact immune system and a mouse model of immunodeficiency that lacked T cells and B cells, two important types of immune cells.
At the end of the experiment, Dr. Martin and his team compared the genes mutated by CRISPR in tumor cells in the control mice to that of the immunodeficient mice. If a given gene was more commonly mutated in the control mice with fully functioning immune systems but not in the immunodeficient mice, this would suggest that the immune system increased selection for cancer cells missing that gene. This also would suggest that the altered gene is related to how cancer cells interact with the immune system.
Dr. Martin and his colleagues identified over 100 such genes. Surprisingly, when the researchers reviewed existing scientific literature on the identified genes, they were among the genes most commonly mutated in cancer patients. Most of these genes fall into the category of
tumor suppressor genes
or TSGs. TSGs function to prevent abnormal cell growth. TSGs that are mutated and lose their function can often lead to cancer.
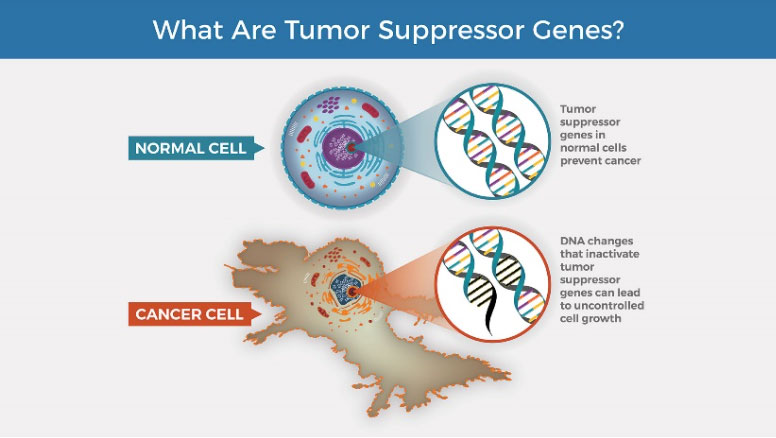
Figure 4. Tumor suppressor genes in healthy cells and cancer cells
[Source: https://visualsonline.cancer.gov/details.cfm?imageid=12495]
In order to determine whether these results were specific to the breast cancer cell line or more generalizable across cancer types, the researchers repeated their experiment using CT26 colon cancer cells, a cell line commonly used in colon cancer research. Again, Dr. Martin and his team developed a library of cancer cells where each entry had a single gene removed and observed how the cancer cells grew in mice with functioning and deficient immune systems. The researchers noticed a remarkably similar result. “We identified many genes whose mutation increased tumor growth in mice with intact immune systems, and many of these genes were known TSGs,” remarked Dr. Martin. However, the specific TSGs that were identified differed between breast and colon cancer cells. The differences in the genes whose mutation increased growth only in the presence of the immune system suggests that different types of cancers use different mechanisms to evade the immune system.
The researchers conducted follow-up experiments on the genes whose mutation increased tumor growth only in the presence of the immune system, including the genes Gna13, Hdac2, and Cul3. The results of these studies confirmed that mutations in these genes led to their loss of function and allowed increased tumor growth in the presence of an immune system.
In addition, the researchers designed similar experiments to test additional known or predicted TSGs, some that had been identified in the previous studies and some that had not. The researchers created a cell library for three distinct cancer cell lines: KP1233 lung cancer cells, 185-3
malignant peripheral nerve sheath tumor cells,
and B16
melanoma
cells. The genes whose mutation increased in abundance in the presence of the immune system included B2m and Hdac2, which were also observed in the 4T1 breast cancer cells and CT26 colon cancer cells.
Some genes were selectively mutated in one cell type but not others. For example, mutations in the Spred1 gene were found only in the B16 melanoma cells, while mutation of the Stk11 gene was unique to the KP1233 lung cancer cells. Overall, the results across five different types of cancer cells support the hypothesis that the immune system exerts selective pressure on tumor cells, which can lead to loss of TSG function.
“One of our key findings is that many of the most commonly mutated TSGs found in cancer patients may also play a role in how the cancer adapts to evade the immune system,” commented Dr. Martin. “The specific genes involved may vary from cancer type to cancer type, which is why it’s so important to have a variety of treatments available based on the genetics of a patient’s cancer.”
To date, Dr. Martin and colleagues have conducted these experiments using only 40% of the genome of cancer cells. In future studies, the researchers plan to expand their focus to include the other 60% of the genome that has not yet been investigated. In addition, Dr. Martin and his team plan to conduct further research into particular genes to determine their specific functions and possible avenues for future treatments.
In the future, Dr. Martin imagines a world where patients can receive more personalized cancer treatment specific to their type of cancer. For example, when a patient receives a cancer diagnosis, their cancer genome would be sequenced, and mutations identified. With these results, doctors could recommend specific treatment, including immunotherapy, that had been shown to work best against cancers with those specific mutations.
Dr. Timothy Martin is a Postdoctoral Fellow in the lab of Dr. Stephen Elledge at Harvard Medical School. Dr. Martin specializes in screening tools for investigating cancer genetics. When not in the laboratory, Dr. Martin enjoys spending time outdoors and attending local sporting events.
This project was done in collaboration with Dr. Rupesh Patel, a former graduate student in the Elledge laboratory now in medical residency at Scripps Green Hospital/Scripps Memorial Hospital in La Jolla, California. Dr. Patel was key in establishing the animal experiments and data analysis.
To Learn More:
- Martin, Timothy, et al. 2021. “The adaptive immune system is a major driver of selection for tumor suppressor gene inactivation.” Science, 373: 1327-35. https://www.science.org/doi/10.1126/science.abg5784
- Brownlee, Christy. 16 Sept 2021. “Immune Escape.” Harvard Medical School News & Research. https://hms.harvard.edu/news/immune-escape
For More Information:
- Elledge Laboratory. https://elledge.hms.harvard.edu/?page_id=63
- Immunotherapy to Treat Cancer. National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
- Immunotherapy. American Cancer Society. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy.html
- The Genetics of Cancer. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/genetics
- Overview of the Immune System. https://www.niaid.nih.gov/research/immune-system-overview
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

