Highlights
A team of researchers developed a novel type of vaccine by engineering an adenovirus-derived dodecamer (called ADDomer) with harmless parts of the chikungunya virus to make a highly potent, heat stable vaccine that does not require refrigeration. These researchers demonstrated that the vaccine candidate provokes the immune systems of animal models to develop antibodies to prevent the chikungunya virus. These experiments validate ADDomer as a potent new vaccine particle. ADDomer can serve as a template for the development of new vaccines. Harmless parts of other viruses can be inserted into ADDomer to develop new vaccines against other viruses.
Are all your immunizations up to date? Did you receive the flu vaccine this year? These are common questions you or your parents might be asked at a visit to the doctor, and they show the important role vaccines play in keeping us healthy. Despite their role in eliminating or significantly reducing the presence of many diseases, there are still many infections for which no cure—and no vaccine—exist. This is why many researchers have spent their careers trying to develop vaccines for new diseases.
Recently, an international research team led by Professor Imre Berger at the University of Bristol has developed a novel type of vaccine that has been shown to prevent the chikungunya virus in animal models—and signals the potential for developing entirely new vaccines in the future. Professor Berger co-founded a company, Imophoron Ltd, with Mr. Frederic Garzoni, to commercialize these new ADDomer vaccines.
In order to understand this research, let’s explore the basics of vaccines and how they support the
immune system
to prevent disease. The immune system is a network of cells, tissues, and organs that prevent and fight infection. One of the many ways the immune system does this is by creating specialized proteins called
antibodies
that can neutralize foreign agents—like bacteria and viruses that cause infection and disease.
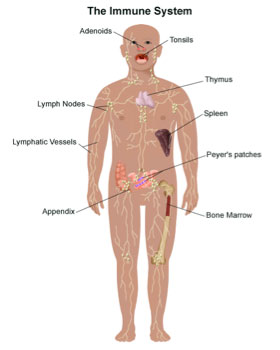
Figure 1. Organs of the immune system.
[Source: https://www.stanfordchildrens.org/en/topic/default?id=all-about-the-immune-system-90-P01665]
One of the reasons that breast milk is so important is that babies receive their first antibodies through their mothers’ milk. As babies grow, they develop antibodies to a variety of micro-organisms in their environment, usually by becoming sick and then fighting off the illness. This is true even as adults: the more infections we fight off, the more types of antibodies our immune system develops.
Some diseases are so strong they can overpower the immune system, causing serious illness or death before the body can create the antibodies needed to fight back. However, if the body was already familiar with the disease and already knew which antibodies to create, the person might not get so sick, or not get sick at all. This is the idea behind vaccinations.
Vaccines
are used across the United States (US) and around the world to limt the spread of infectious diseases. In many US states, parents must show documentation that their children have received all their vaccines in order to attend public schools, with few exceptions. For example, in Massachusetts, required childhood vaccinations include
diphtheria,
hepatitis B,
measles,
mumps,
pertussis
(whooping cough),
rubella,
and
varicella
(chicken pox). That’s a lot of vaccines! All these diseases used to be much more common, and their occurrence is now rarer due to widespread vaccination.
Clearly vaccines help prevent and control the spread of disease—when they exist. However, there are many diseases for which scientists still have no vaccine, let alone treatment. As the climate changes, the range of infectious diseases and the animals that carry them are shifting, creating the possibility for more people to be exposed to more disease. This is a trend that will only get worse with time.
One example of a disease increasing in range is the
chikungunya virus,
which was once only a tropical disease and has now spread into Europe and the Americas. The chikungunya virus is carried by the same species of mosquito that carries
dengue fever
and the
zika virus.
As the range of this mosquito widens, so too will the spread of disease.
The most common symptoms of the chikungunya virus are fever and joint pain, which usually begin within one week of being bitten by an infected mosquito. Other symptoms of the disease include headache, muscle pain, joint swelling, and rash. Most people who are bitten by an infected mosquito will develop symptoms. Although the disease is usually not fatal, some symptoms may persist for months. Infants, the elderly, and people with other diseases are at a higher risk for developing complications from chikungunya. There is no treatment or cure for the disease. “The development of treatments for chikungunya and other infectious diseases is critical,” explained Professor Berger. “But in the long run we need vaccines to prevent further spread of the disease.”
To develop a vaccine for the chikungunya virus, Professor Berger teamed up with
virologist
Dr. Pascal Fender at the French National Centre for Scientific Research in Grenoble, France. To be successful, vaccines must be easy to produce in large quantities at low costs. Ideally, they would also be
thermostable
to facilitate production, storage and transport – today, most vaccines require refrigeration and significant quantities are lost because they are not refrigerated during transport to where they are needed.
In previous work, Dr. Fender had studied
human adenovirus
vectors,
which are widely used in
gene therapy.
Research showed that one protein component of human adenovirus formed on its own a large virus-like particle, or VLP, in the test tube. VLPs resemble a virus from the outside, but in contrast to viruses do not contain any genetic material so cannot replicate and become infectious in the body. VLPs are made of many copies of identical building blocks that are highly repetitive.
One day, Dr. Fender placed a test tube of this VLP in his lab coat and forgot about it. Several months later, when he found the test tube in his coat pocket, he was surprised to learn that the VLPs were still intact. This meant the VLPs were stable at room temperature and did not need refrigeration. This discovery is how the scientists realized that these particular VLPs might be a good candidate to function as the building block for a novel type of vaccine. They called this building block ADDomer for Adenovirus-derived dodecamer, referring to its characteristic shape resembling a large dodecahedron.
Dr. Fender and Professor Berger hypothesized that if they could engineer ADDomer by decorating it with harmless bits and pieces
(epitopes)
of a dangerous disease-causing virus, they might be able to make a highly potent, thermostable vaccine. For chikungunya virus, their goal was to engineer ADDomer so that it would contain just enough bits and pieces of the chikungunya virus on the outside to provoke the immune system to develop antibodies. These antibodies would then be ready to neutralize the real chikungunya virus if it arrived, providing protection against the disease.
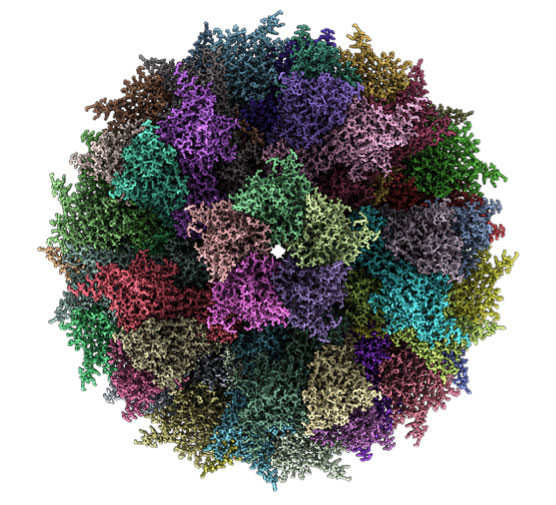
Figure 2. The ADDomer virus-like particle (VLP), seen in the cryo-electron microscope. ADDomer can be engineered to mimic a virus, but, in contrast to real viruses, cannot multiply in the body and is thus entirely safe. ADDomer comprises many identical building blocks (colored differently in this image).
[Source: University of Bristol]
There were many possibilities for vaccines these researchers might create based on the ADDomer. In order to identify the best structure for a possible vaccine, Professor Berger and Dr. Fender joined forces with Professor Christiane Schaffitzel to use a powerful new imaging tool called
cryo-electron microscopy
or Cryo-EM. Cryo-EM allowed the researchers to look deeply at the structure of each possible vaccine molecule, down to the individual atoms. By collaborating with Bristol’s IT specialists Christopher Woods and Matt Williams, along with colleagues from the
cloud computing
company Oracle, the researchers were able to identify the best ways to insert epitopes into the ADDomer in much less time—and at a lower cost—than has ever been possible before.
Once the researchers identified the candidate vaccines, they engineered them in the laboratory. The very first experiments tested the molecules in a test tube to make sure they were stable at room temperature. Next, the researchers exposed immune cells to their candidate vaccine in the petri dish to see how the cells would respond. By doing this, the researchers demonstrated the vaccine candidate provoked the appropriate response in immune cells.
The next step was to inject the chikungunya vaccine candidate into mice and test their blood for antibodies that could potentially fight off the disease. The inoculated mice rapidly developed very high levels of antibodies specific for the chikungunya epitopes decorating the vaccine, compellingly validating the ADDomer as a potent new vaccine particle.
“We are so thrilled with these results,” commented Professor Berger. The vaccine candidate for chikungunya virus is easy to produce and distribute, and it doesn’t require refrigeration. According to the studies conducted so far, the vaccine candidate is working. Next steps for the chikungunya vaccine candidate are testing in larger animal models that are exposed to the chikungunya virus. If those test results continue to be positive, initial trials can begin in humans.
“The best part about this vaccine candidate is that it is not limited to chikungunya,” added Mr. Frederic Garzoni. “We can develop many new ADDomer-based vaccines using the same VLP core, and we are wasting no time in doing just that.”
Imre Berger is Professor of Biochemistry and Director of the Max Planck-Bristol Centre for Minimal Biology in Bristol, England. His research focuses on the use of
synthetic biology
and innovative structural and computational tools to transform molecular biology. When not in the laboratory, Imre and his favorite collaborator, Christiane Schaffitzel, enjoy traveling and spending time with their two wonderful children, most preferably on the sunny beaches of the French Atlantic coast.
Pascal Fender is Director of Adenovirus research at the Centre National de Recherche (CNRS) in Grenoble, France. When not in the lab, Dr Fender enjoys hiking and spending time with his family in the beautiful mountains surrounding Grenoble.
Frederic Garzoni is CEO and Director of Imophoron Ltd located at UnitDX, Bristol’s deep tech incubator. When not running the company, Mr. Garzoni enjoys soccer, cross fit, and spending time with his family.
For More Information:
- Vragniau C, et al. Synthetic self-assembling ADDomer platform for highly efficient vaccination by genetically encoded multiepitope display. Scientific Advances 2019;5:eaaw2853. https://advances.sciencemag.org/content/5/9/eaaw2853
To Learn More:
- Imophoron. https://unitdx.com/novel-vaccine-technology-interview-fred-garzoni-imophoron/
- Overview of the Immune System. https://www.niaid.nih.gov/research/immune-system-overview
- Vaccines and Immunizations. https://www.cdc.gov/vaccines/index.html
- Vaccines. https://www.vaccines.gov/
- Vaccines. https://www.fda.gov/vaccines-blood-biologics/vaccines
- Making Vaccines. https://www.pbs.org/wgbh/nova/body/making-vaccines.html
- What are Viruses? https://www.livescience.com/53272-what-is-a-virus.html
- Chikungunya Virus. https://www.cdc.gov/chikungunya/index.html
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

