|
Medical researchers use animal models to understand the development and progression of human diseases. Rodents, particularly mice, are among the most popular types of animals used as models in medical research. Mice make good animal models of human disease because they are physiologically similar to humans, they reproduce quickly, and they are relatively easy to care for. There are mouse models for thousands of human diseases including diabetes, heart disease, and many types of cancer.
Still, there are important physiological differences between mice and humans that can make medical research particularly difficult. A good example of this is a disease known as Duchenne Muscular Dystrophy.
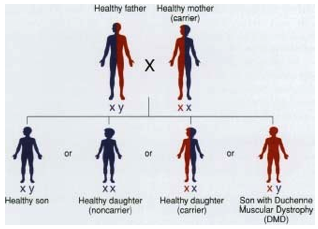
Sons have a 50-50 chance of inheriting the disorder from their
mother if she carries the gene for it on one of her X chromosomes.
Duchenne Muscular Dystrophy (DMD) is a severe, chronic muscle wasting disease caused by a mutation in a gene called the DMD gene located on the X chromosome. DMD is an example of an X-chromosome-linked disease that is usually inherited from mother to son. Females have two copies of the X chromosome, so one mutant copy will not cause them to have DMD. A mother can, however, pass on the mutant X chromosome to her son. If her son inherits the X chromosome with the mutant DMD gene, he will get the disease because he only has one X chromosome. DMD affects approximately one in every 3,600 males.
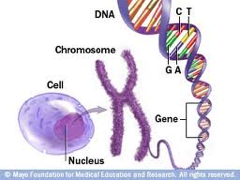
The relationship among the cell, the nucleus,
a chromosome and a gene.
A reminder of what genes do: "Genes tell a cell how to make proteins. Roughly speaking, each gene is a set of instructions for making one specific protein. Proteins are a diverse group of large, complex molecules that are crucial to every aspect of the body's structure and function."
The DMD gene encodes for a large protein known as dystrophin. Dystrophin is part of a group of proteins that strengthen muscle fibers and protect them from injury as muscles contract and relax. Patients with DMD donít have any dystrophin, so their muscle cells are easily damaged by the contraction and relaxation of normal muscle movement. DMD babies may look healthy for a few months, but pretty soon their bodies canít keep up with the extent of muscle damage, and the muscles start to weaken. People with DMD have usually lost their ability to walk around the age of 10 years and are confined to a wheelchair. Most patients will die by the time they are 20 or 30 years of age because of muscle damage to muscles such as the heart or those that control breathing.
Modeling
One of the first steps for researchers wanting to better understand a disease and test treatments is to make a mouse model that mimics the progression of human disease. It has been known for over twenty-five years that mutations in the DMD gene cause DMD in humans, but mice with the exact same mutation donít develop the disease. This has puzzled researchers for quite some time. Some hypothesized that mice did not develop DMD because they were too small or they didnít live long enough. Dr. Helen Blau of the Stanford School of Medicine was not satisfied with those answers. "I just thought there might be something more to it," she said. Dr. Blau has spent her career trying to understand muscle cells and their role in the development of DMD. Although mice with the DMD mutation did not develop the disease, they exhibited muscle damage just like humans with DMD. Unlike their human counterparts, though, the mice could keep up with the damage. There was a normal cycle of damage and repair in DMD mice that you would see in normal humans but not in people with DMD. So Dr. Blau and her team of researchers started to think about the physiological differences between mice and humans to try to find an explanation.
One big difference between mice and humans is telomere length.Telomeres are regions of repetitive DNA at the ends of chromosomes that protect chromosomes from degrading during replication. Every time a cell divides, a small portion of DNA is lost from the ends of each chromosome. The longer the telomere is, the more times the cell can divide before a division will result in damage to chromosomes. Cancer cells, for example, have very long telomeres.
What It Means to Test a Hypothesis
Dr. Blau hypothesized that mice with a mutation in the DMD gene do not develop DMD because their muscle stem cells have longer telomeres. The muscle stem cells are critical to repairing muscle damage. Muscle stem cells become muscle cells when needed. When muscle cells die as a result of DMD, muscle stem cells become new muscle cells to repair the damage. Stem cells with longer telomeres can undergo more cell divisions. This means that there will be an increased number (reservoir) of muscle stem cells available to replace damaged muscles.
In order to test this hypothesis, Dr. Blau, and her team, developed a mouse model that had both a mutation in the DMD gene and stem cells with shortened telomeres like humans. These mice are called double-mutant mice. She then compared these double-mutant mice (mice with the mutant DMD gene and short telomeres) to mice with just the mutant DMD gene and normal length telomeres as well as to wild type (control) mice (mice without either mutation). Dr. Blau wanted to know whether mice with the DMD mutation and shorter telomeres would develop DMD. Dr. Blau and her team of researchers performed several tests on these three groups of mice in order to determine whether the shortened telomere length contributed to the development of DMD. Importantly, she looked only at male mice because generally only males will get the disease.
In the first set of experiments, Dr. Blau and her team analyzed tissue samples from all three groups of mice looking for specific indicators of muscle cell damage. One of these "markers" is the enzyme called creatine kinase, which plays an important role in energy storage in muscle cells. High levels of creatine kinase in the blood indicate muscle damage. The results showed the double-mutant mice (with short telomeres) had high levels of creatine kinase compared to the mutant mice (with longer telomeres) and the wild type mice. These levels were similar to those found in humans with DMD. These results indicated the shortened telomeres contributed to the development of DMD in mice.
In another set of experiments, Dr. Blau assessed the performance of muscle cells by having the mice run on a treadmill and measuring the time until exhaustion. Double-mutant mice (with short telomeres) became exhausted much more quickly than mutant mice (with long telomeres) or wild type mice. The muscle performance of the double-mutant mice continued to decrease with age. Dr. Blauís team also measured muscle force and time to fatigue (tetanus). Compared to the other two groups of mice, the double-mutant mice (with shortened telomeres) had a much weaker performance that decreased as the mice aged. Analysis of muscle cells taken from the double-mutant mice provided further evidence of muscle damage by microscopy.
Telomere Length - More Hypothesis Testing
All of these results provided strong evidence that shortened telomeres of muscle stem cells in mice with the mutant DMD gene caused the development of DMD. To further test this hypothesis, Dr. Blau performed stem cell transplantation on a group of double-mutant mice. If the reason the double-mutant mice were developing DMD was because they were no longer making enough stem cells, it would be expected that the addition of new stem cells would reverse the degeneration. By contrast, stem cells from mice with DMD would not adequately regenerate the muscles. Indeed, this is what Dr. Blau found.
The muscle wasting and disease progression in the double-mutant mice as a result of a mutant DMD gene and shortened telomeres closely mimic DMD in humans. These findings suggest a new mouse model for DMD that researchers will be able to use to better understand the disease and explore treatment options. Importantly, this research shows that DMD is not just a genetic disorder caused by the deficiency of dystrophin, but it is a stem cell disease as well. There arenít enough stem cells to repair the damage caused by the lack of dystrophin. "These are very exciting results," remarks Dr. Blau. "This is a major change in the way we think about this disease" explains Dr. Blau.
Now that a good mouse model for DMD has been established, the next step for Dr. Blau is to try to treat the mice. This would involve treatments for both the lack of dystrophin as well as a lack of stem cells. In the future, Dr. Blau envisions a combination of gene replacement and stem cell replacement to treat this debilitating disease.
Beyond DMD
There are also applications to this research beyond DMD. For example, when older people break a bone, such as a hip, and are hospitalized, their muscles weaken so quickly that sometimes they cannot walk again. Dr. Blauís research on DMD could lead to treatments that would stimulate muscle growth and help patients become active again after injury.
"This has been a long but incredibly rewarding process," comments Dr. Blau. "Nearly three decades ago we noted that DMD muscle cells had a growth defect," however, only in the last few years did Dr. Blau and her team figure out how to create a mouse model that successfully mimicked the progression of DMD. "I took a lot of risks with this research," she said. "But it has certainly paid off." Dr. Blau hopes to see her work lead to an improvement in the lives of children affected by DMD, and help them and their families.

Stanford University
Dr. Helen Blau is the Donald E. and Delia B. Baxter Professor, in the Department of Microbiology and Immunology at the Stanford School of Medicine and the Director of the Baxter Laboratory for Stem Cell Biology at the Stanford Institute for Stem Cell Biology and Regenerative Medicine. Her research focuses on tissue regeneration with particular emphasis on stem cells, including muscle stem cells and Duchenne Muscular Dystrophy. When not in the laboratory, Dr. Blau enjoys traveling, playing music, skiing, scuba diving and spending time with her family.
For More Information:
- 1. Sacco, A. et al. 2010. "Short Telomeres and Stem Cell Exhaustion Model Duchenne Muscular Dystrophy in mdx/mTR Mice." Cell, 143: 1059-1071.
To Learn More:
Rebecca Kranz with Andrea Gwosdow, Ph.D. Gwosdow Associates
A note about Wikipedia from Wikipedia: "Wikipedia cannot guarantee the validity of the information found here. The content of any given article may recently have been changed, vandalized or altered by someone whose opinion does not correspond with the state of knowledge in the relevant fields. Note that most other encyclopedias and reference works also have similar disclaimers." http://en.wikipedia.org/wiki/Wikipedia:General_disclaimer.
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

