| |
The ice cream truck flashes its lights and plays Yankee Doodle as it pulls up next to the playground. All the kids come running with their parents in tow. The kids want sugar – and their parents don’t want them to have it. Why? Because parents know that when their children eat too much sugar they get hyped up and bounce off the walls for a while: then they crash. Why does this happen?
The sugar found in candies and ice cream is called glucose. It is already in its simplest form, so your body does not need to break it down before using it. That means that the sugar gets used up quickly, giving you a rush of energy, a “sugar high.” It also means that when the sugar is gone, there is the inevitable crash and you feel exhausted.
But millions of Americans cannot get that sugar high. That’s because they lack the ability to produce insulin, the hormone that controls the uptake of glucose from the blood into the cells and organs of the body. Without insulin, these people are unable to use the glucose they eat. If left untreated, people without insulin would not survive, because high levels of blood glucose cause many harmful effects. The condition of lacking insulin is known as type-1 diabetes. The current data indicate that about 15,000 young people in the United States are diagnosed with type-1 diabetes each year.
Diabetes
Type-1 diabetes is just one form of diabetes mellitus, a disease that has been known for thousands of years. A person with this condition would drink water and then urinate a sweeter liquid. The Romans called it diabetes mellitus form the Latin words diabetes meaning to pass through and mellitus meaning honey or sweet. Diabetes mellitus is a group of diseases all characterized by hyperglycemia, or high blood sugar levels. The two most common forms of diabetes mellitus are type-1 and type-2, but there are other forms of the disease as well.
Type-2 Diabetes
The bodies of people with type-2 diabetes produce insulin but the insulin cannot be used. This may be due to the lack of insulin receptors or other malfunctions along the insulin pathway. Type-2 diabetes has been associated with obesity and high-sugar diets; it is traditionally characterized by onset later in life. But recently, more and more children are being diagnosed with type-2 diabetes, possibly due to an increase in the rate of childhood obesity.
People with type-1 diabetes simply do not produce insulin. This is the result of an autoimmune problem: the person’s own immune system attacks the cells in the pancreas responsible for insulin production: they are known as β-cells (beta-cells). About 2% of the normal pancreas is made of cells called the Islets of Langerhans Inside each Islet are several types of cells, namely α-cells and β-cells.
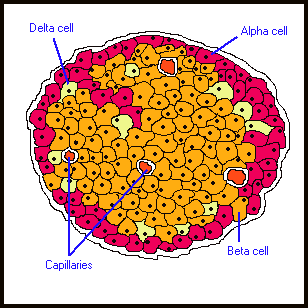 The β-cells make up about 70 - 80% of Islet cells and are responsible for the production of insulin. In type-1 diabetes, the person’s own immune system targets healthy β-cells. The β-cells are destroyed by the immune system and cannot produce insulin, resulting in the condition of high blood sugar and diabetes. The β-cells make up about 70 - 80% of Islet cells and are responsible for the production of insulin. In type-1 diabetes, the person’s own immune system targets healthy β-cells. The β-cells are destroyed by the immune system and cannot produce insulin, resulting in the condition of high blood sugar and diabetes.
Insulin
Insulin is the most famous of the pancreatic hormones that control blood glucose levels, but it does not work alone. Insulin and glucagon, another pancreatic hormone, work in opposition to regulate blood glucose in the body. Insulin stimulates the uptake of sugar from the blood; glucagon stimulates the release of sugar back into the blood stream. In a healthy person, when blood glucose levels get too high, the body is stimulated to produce more insulin and uptake glucose from the blood. On the other hand, when blood glucose levels get too low, the body is stimulated to produce more glucagon which, in turn, causes the body to release glucose into the blood stream. Lacking insulin, this complex regulatory system cannot function.
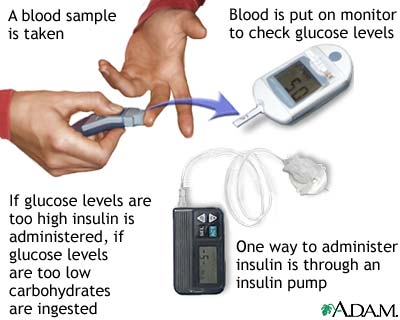
Patients with type-1 diabetes must complete the system by giving themselves insulin injections whenever blood glucose levels get too high. This involves measuring blood glucose levels. To measure blood glucose levels, people with diabetes prick their finger to get a drop of blood, which is then put in a machine that measures the blood glucose level. People with diabetes must do this several times per day. If their blood glucose levels are too high, they give themselves an insulin injection to stimulate the uptake of glucose from the blood. This can be a complicated process, though. Insulin is needed at some times but not at others. For example, insulin is needed after eating so the body can use the sugar that has just been consumed, but not before or during exercise. Right now this is the only way we know to control type-1 diabetes.
As a clinician Dr. Roger Unger saw hundreds of patients with type-1 diabetes in the VA North Texas Health Care System and at the University of Texas Southwestern Medical Center. For unconscious patients who had overdosed with insulin, he would sometimes prescribe glucagon to restore consciousness by correcting the low glucose level. Working with patients with type-1 diabetes, Dr. Unger saw how cumbersome and painful the treatment is for this disease, and he began looking for alternatives to the classic treatment of insulin injections.
The Leptin Connection
One possibility Dr. Unger came across was the hormone leptin, which was discovered in 1994 by Dr. Jeffrey Friedman of the Howard Hughes Medical Institute. Dr. Friedman studies the relationship between food intake and the regulation of body weight. He found that leptin is produced by fat cells and plays a key role in body weight regulation. Leptin was reported to lower glucose levels in normal rodents (Koyama et al. 1997) and Dr. Unger thought that leptin might play a role in type-1 diabetes as well.
He began to test his hypothesis using non-obese diabetic mice (NOD mice), which are the most commonly used mouse model for type-1 diabetes. NOD mice have dangerously high blood sugar levels because their beta cells have been destroyed. He and his colleagues injected some NOD mice with a leptin-adenovirus while others served as the control group. Surprisingly, the blood sugar levels of the leptin-treated mice returned to normal several days after the injection and remained normal for over 150 days. The untreated control group died of insulin deficiency. “This was an incredible result,” Dr. Unger remarked. “Insulin patients currently have to take insulin several times per day. These mice retained normal blood sugar levels for months.” Dr. Unger stresses, though, that these results are preliminary using adenovirus-leptin and cannot be taken as indicative of the effect of leptin in humans. These initial findings are a huge shock to the field of diabetes and have added a new perspective to the classical model of insulin as the only hormone involved in type-1 diabetes.
To see whether these results could be reproduced in other animals, Dr. Unger and his team repeated these experiments in rats in which type-1 diabetes had been chemically induced. The treated rats were given leptin by adenovirus gene transfer and the control group was not. The rats that received leptin injections improved to a normal state and remained that way for several months. The control rats died in diabetic coma.
The next step for the Unger lab was to determine why leptin had such a profound effect on both the mice and rats. As you recall, glucagon works together with insulin to regulate blood sugar levels. Since the NOD mice and chemically-induced rats do not produce insulin, Dr. Unger wondered whether leptin altered blood glucose levels by changing the amount of glucagon in the blood. To test this hypothesis, the researchers measured the amount of glucagon in each blood sample. Glucagon was very high in untreated diabetics but in leptin-treated animals it was normal. He concluded that leptin brought blood glucose levels back to normal by inhibiting glucagon. This lowered blood sugar levels in the treated animals, but not in the controls.
The Work of Insulin
Another function of insulin is to help blood glucose actually enter the cells. Insulin acts by binding to something called the insulin receptor on the surface of the cell: this allows blood glucose to enter. Without insulin present to bind to its receptor and allow glucose into cells, it was unclear how leptin could lower blood glucose levels.
To determine if something else (specifically, an insulin-like hormone) might be mimicking insulin, Dr. Unger measured the protein called insulin-like growth factor-1 (IGF-1). IGF-1 can interact with the insulin receptor and, when stimulated, can produce some of the same intracellular signals as insulin. Dr. Unger and his team found that leptin-treated animals had increased amounts of IGF-1, and control animals did not. Dr. Unger hypothesizes that leptin may be exerting its insulin-like effect by acting through IGF-1.
Treatments - If Any - May be Decades Away
Given the astounding results of reversing hyperglycemia in rats and mice, it is possible that one day leptin may be an alternative to insulin injections. “But that time is still very far in the future,” cautions Dr. Unger. While he is excited about his new findings, he is reluctant to speak of any new treatments. “These experiments were done in rodents. While rodent and human biology are similar in many ways, they are also very different.” Similar tests would have to be done in larger mammals and if leptin succeeded in normalizing blood glucose levels in those animals, it would move through rounds of clinical trials. “The process will take years and any new treatment could be decades away.” Still, Dr. Unger’s findings have turned diabetes research upside down and opened the field to a whole new range of possibilities. For now Dr. Unger is conducting more experiments to determine the leptin pathway and figure out exactly how leptin works to exert its insulin-like effects.
Dr. Roger Unger is a Professor of Internal Medicine at the University of Texas Southwestern Medical Center and the VA North Texas Health Care System, as well as a member of the National Academy of Sciences and the American Academy of Arts and Sciences. He was the Director of the Touchstone Center for Diabetes Research between 1986 and 2007. Dr. Unger began his career as a practicing physician working with diabetes patients before he began research in the field. His work over the years has included research on insulin and glucagon mechanisms of function and most recently his research has focused on the role of leptin in the body.
Dr. Unger emphasizes that none of his research would have been possible without his dedicated team of researchers at both the Dallas VA Medical Center and UT Southwestern Medical Center.
For More Information:
- Unger, Roger. Noninvasive tracking of gene expression by reporter transgene imaging. Proceedings of the National Academy of Sciences 2008; 105(35): 12641-2.
- Yu, Xinxin et al. Making insulin-deficient type-1 diabetic rodents thrive without insulin. Proceedings of the National Academy of Sciences 2008; 105(37): 14070-75.
To Learn More:
Written by Rebecca Kranz with Andrea
Gwosdow, Ph.D. Gwosdow
Associates
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Research led by Dr. Roger Unger has shown in rodents that leptin, a hormone produced by the body's fat cells, lowers blood glucose levels. The discovery may lead to a treatment option other than insulin for humans with type-1 diabetes.
Photo courtesy of UT Southwestern Medical Center.

Leptin research at the University of Florida
Video courtesy of Youtube.com

To hear Dr. Jeffrey Friedman talk to a group of high school students about the discovery of leptin and its connection to obesity, click here
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

