Highlights
- A cellular model was developed to study color vision by blue, red, and green cone cells, and their regulation. First, stem cells were transformed into retinal cells by adding chemicals that caused the cells to develop into cone cells. The pattern of development was blue cone cells developed first, and red and green cells developed later, just like in the human retina.
- The researchers identified thyroid hormone as a key regulator of cone cells. When a gene that responds to thyroid hormone was removed, the organoids only had blue cone cells! When extra thyroid hormone was added, the organoids developed only green and red cone cells, no blue ones.
- The results indicate that thyroid hormone is important for the development of vision.
If you look up close at the eyes of a fruit fly, you will see that its big eyes are actually made up of hundreds of small eyes. There are two types of these small eye units, and they are distributed randomly. “I was so fascinated by this,” explains Dr. Robert Johnston, Assistant Professor at Johns Hopkins University, “because in
developmental biology,
you almost never want something random.”
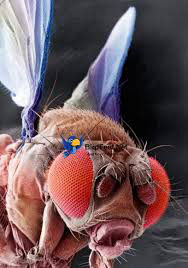
Figure 1. Fruit fly with red eyes.
[Source: https://tinyurl.com/yc6d7kjk]
For example, if you are fortunate enough to have two hands with five fingers each, you will see that the right and left sides are nearly symmetrical. This is not random but rather very intricately programmed so that each type of cell and each part of the body develops at the appropriate time.
To understand this programming, let’s see how the mammalian eye works. The outer portion of the eye includes the
cornea,
the
iris,
and the
pupil.
The cornea is the white layer you see in the front of the eye, and the iris is the ring around the pupil that can come in different colors like brown, blue, or green. The pupil is actually a hole leading further back into the eye. The cornea helps bend light rays as they move through the pupil, and the iris opens and closes the pupil to allow more or less light in. Light that enters through the pupil travels through a thick fluid until it hits the
retina
in the back of the eye. The retina is a think layer of specialized nerve cells called
photoreceptors
that capture light rays and convert them into electrical impulses that go to the brain. This is how vision works.
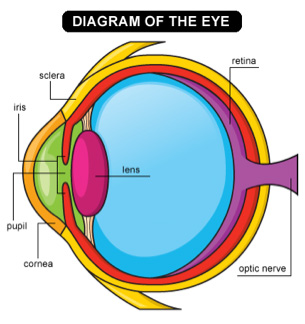
Figure 2. A diagram of the eye.
[Source: https://tinyurl.com/yda76h93]
The retina has two main types of photoreceptors, called
cone cells
and
rod cells,
based on their very distinct shapes. Rod cells are located in the periphery of the retina and assist with
peripheral vision,
night vision, and with capturing movement. Cone cells are most concentrated in the center of the retina, in an area known as the
macula.
These cells process our central vision including color vision. Humans have three types of cone cells that allow us to see color: blue cone cells, red cone cells, and green cone cells.
Dr. Johnston studies how different photoreceptor cells in the eye develop. “There are many types of diseases of the eye,” explains Dr. Johnston, “but it has been difficult to find treatments for color vision because there are no good
animal models
for human color vision. Common animal models see color very differently from humans. For example, mice see only two colors, green and blue, whereas fish see four colors. Moreover, the patterning of the cells in the eyes are very different.”
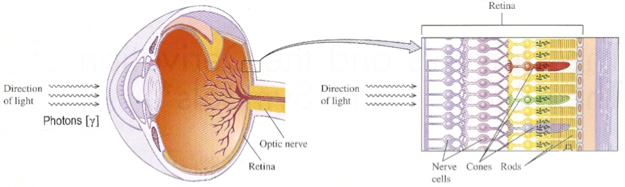
Figure 3. A closer look at the retina and photoreceptors in the human eye.
[Source: https://tinyurl.com/y7krht2r]
Eye in a Dish
Given the lack of animal models to study color vision, Dr. Johnston and his team set out to develop one. At one fateful meeting, Dr. Johnston saw a talk given by Dr. Yoshiki Sasai, in which he reported that he had been able to grow human retinal tissue in a dish from stem cells! Dr. Johnston imagined that he could use this same protocol to understand how human retinal cells develop.
All of the cells in our body begin as stem cells that need to be directed during our development in the womb to make all the different kinds of cells in our body. These stem cells don’t have any specialized function, but they can be stimulated to become any type of cell through a variety of chemical signals. In order to make stem cells in the laboratory become cone cells, Dr. Johnston and his team would need to introduce the appropriate chemical signals.
The researcher who performed these experiments in Dr. Johnston’s laboratory is Kiara Eldred, a graduate student and Howard Hughes Medical Institute Gilliam Fellow for Advanced Study. Since Dr. Johnston had not done any previous work in human stem cells, Kiara went to the laboratory of one of Dr. Johnston’s colleagues, Dr. Donald Zack to learn the experimental steps from a talented former Postdoctoral fellow in the laboratory, Karl Whalin. Once she had been trained, Kiara ordered all the stem cells, chemical signals, and other equipment she would need to start the experiments.
Human eyes take about nine months to develop. Once Kiara began the process of stimulating stem cells to become retinal cells, she had to wait about 200 days for the retina to develop.
The stem cells begin their journey in a plastic dish in a liquid that contains all the nutrients they need to grow, called growth media. On Day 1 of the experiments, Kiara gathered the cells in a round bottom well and let them fall to the bottom in clumps. The clumps of stem cells would form human retinas over the course of the next 200 days.
For the first 40 days of the experiment, Kiara added chemical signals to the media to stimulate the stem cells to become photoreceptors. Every day Kiara changed their growth media so they had fresh nutrients, with the proper chemical signals for the day, and observed the pre-retinal clumps to make sure they were growing properly. At Day 8, Kiara had to physically cut the clumps into different pieces to separate the developing retinas from one another.
After 40 days of stimulation with chemical signals, the cells were transferred to a regular medium where they could continue growing, and the growth media only needed to be changed every other day. At this point, Dr. Johnston and his team call these clumps of cells “organoids,” because they are organ-like.
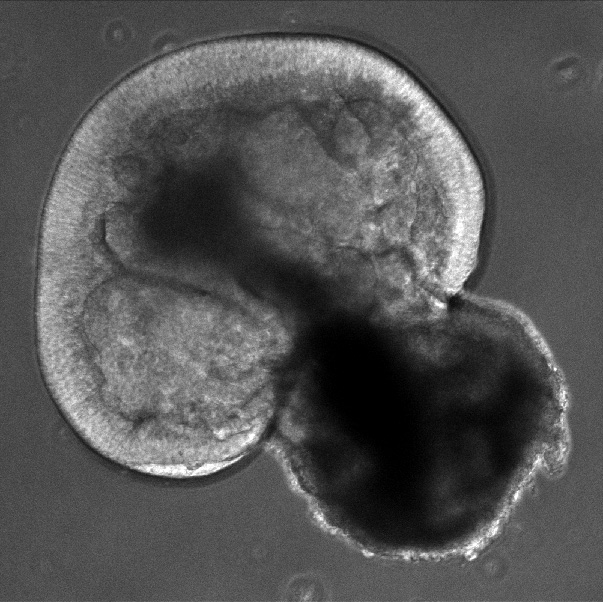
Figure 4. Day 43 organoid: Bright field image at 100x magnification of a live organoid growing in a dish. The outer layer of the cup shape is where the photoreceptors will grow, in the future, they have not been generated at this time. [Photo courtesy of Kiara Eldred]
Kiara added proteins to the developing organoids that would cause the different types of cone cells to fluoresce different colors under the microscope, so blue cone cells would become blue, red cone cells would become red, and green cone cells would become green.
Experimenting with Organoids
The goal of the first set of experiments was to verify that the system Kiara developed actually worked. In order to be similar to the human retina, the retinal organoid would need to produce blue, green, and red cone cells. Over the course of the 200 days that the organoids were growing, Kiara tested them periodically and counted how many blue, red, and green cone cells had developed. She did this by looking at the cells under the microscope and hand-counting the cells based on their fluorescent colors.
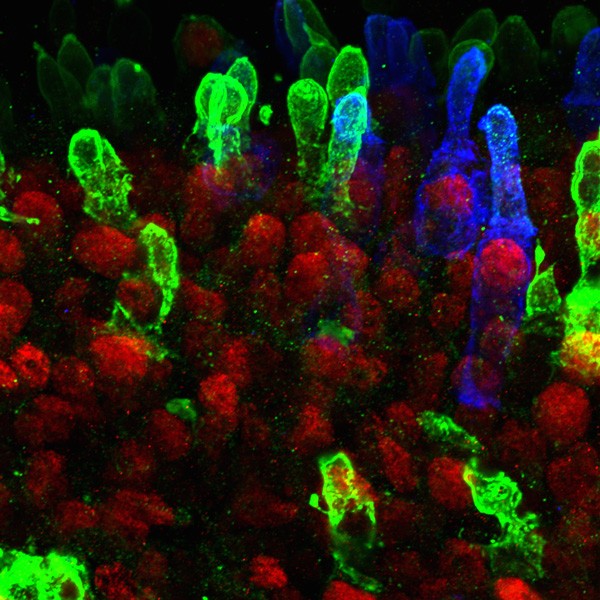
Figure 5. Day 322 organoid: 600x magnification confocal image of the organoid retinal tissue. Blue: blue cone cells. Green: both red and green cone cells. Red: Transcription factor CRX (cone-rod-homiobox transcription factor) that is expressed specifically in the nucleus of photoreceptors. [Photo courtesy of Kiara Eldred]
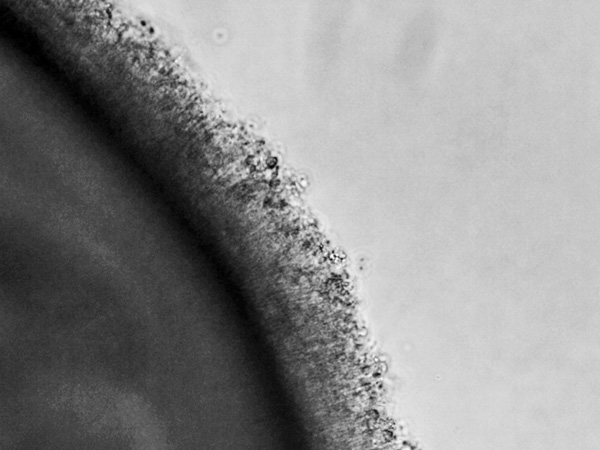
Figure 6. Day 330 organoid: Bright field image at 300x magnification of a live organoid growing in a dish. The mature photoreceptors are growing out of the organoid, sending their outer segments into the media. The outer segment of the photoreceptor is where the opsin proteins or photopigments that sense specific spectrums of light are located. [Photo courtesy of Kiara Eldred]
After doing this over and over, Kiara noticed a pattern. In the organoids, the blue cone cells developed first, and the red and green cells developed later, the same order they develop in the human
retina. “This verified the system that we were working with,” comments Kiara. “We could now use this model to study the development of the cone cells.”
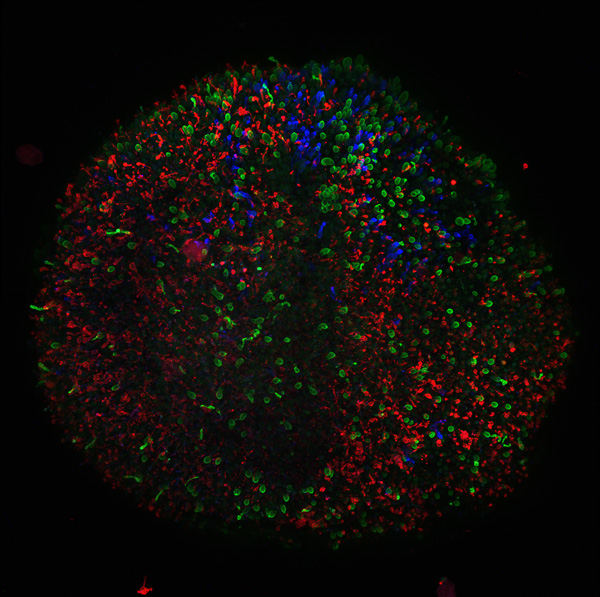
Figure 7. 361 organoid: 200x magnification confocal image of the organoid retinal tissue. Red: rod cells, the photoreceptors that allow us to see in low light conditions. Blue: blue cone cells. Green: both red and green cone cells. [Photo courtesy of Kiara Eldred]
The next step was to determine what was controlling the development of the blue cells first and the red and green cells later. To do this, the researchers turned to the scientific literature and other animal models of vision. They found that
thyroid hormone
was involved in the development of vision in both mice and fish. Researchers had also found that a receptor for thyroid hormone, thyroid hormone receptor beta (Thrβ), was necessary for developing green cones in mice.
In order to determine whether thyroid hormone and Thrβ might be involved in the development of vision in human retinas as well, Kiara used the
CRISPR
(Clustered Regularly Interspaced Short Palindromic Repeats)/Cas9 system to delete Thrβ in stem cells, which she could then grow into a new batch of retinal organoids.
Kiara noted a dramatic change in the organoids when the gene for thyroid hormone was removed. “It was amazing,” recalls Dr. Johnston. “These organoids only had blue cone cells!” In other words, without Thrβ, the red and green cone cells never developed, only the blue ones. Next, Kiara added extra thyroid hormone to increase signaling in this pathway. This time, the organoids developed only green and red cone cells, no blue ones.
From these findings, Dr. Johnston and Kiara knew that thyroid hormone must be involved in the development of the different cone cells. But these cells were in a dish—there was no
thyroid gland
to produce thyroid hormone. So how could thyroid hormone be controlling the development of these cells?
The researchers knew that the media the organoids grew in contained small amounts of thyroid hormone. This suggested that over time the retina itself must control absorption of the hormone. In other words, the retina could use thyroid hormone from the media to develop red and green cone cells even without a connection to the thyroid organ.
To confirm what they were seeing, Kiara used a technology called
RNA sequencing.
This technique looks at all the sequences of RNA that are expressed at a given time in the cell. From this information, the researchers infer which proteins are active. Kiara ran this test at various points during the development of the retina. Through these experiments, she found that proteins expressed early in development were related to the degradation of thyroid hormone, leading to blue cone cells. Proteins expressed later in development were related to the activation of thyroid hormone, leading to red and green cone cells.
Other scientific literature also supports the finding that thyroid hormone is important for the development of vision in humans. One study found that premature babies have a higher probability of developing color vision deficiencies. This could be because the babies aren’t receiving the high levels of thyroid hormone that their mothers produce during pregnancy, which interrupts the development of their vision. Similarly, people with overactive thyroids, a condition known as
Graves’ disease,
report color vision deficiencies as their symptoms progress. This could be because too much thyroid hormone leads to the loss of blue cone cells. All of this means that thyroid hormone is not only important for the development of vision in babies, but it may also be important for maintaining proper vision throughout the lifespan.
Future Work
The next steps for Kiara and Dr. Johnston are to better understand the mechanisms behind the development of the cone cells. They have observed that blue cone cells are formed first, and that the progenitors of red and green cells, which the researchers call red-green cells, are formed later. An additional mechanism occurs that helps the red-green cone cells become either red or green. The researchers know that the development of different cone cell populations is highly regulated, so the question remains whether they can alter the system. Do red and green cells randomly decide which to become? Or are there other mechanisms behind this differentiation? “That’s the goal of our next experiments,” explains Dr. Johnston.
Dr. Johnston also has future experiments planned that directly relate to the development of treatments for human diseases of the eye. For example,
macular degeneration
is the leading cause of blindness across the world. Recall that the macula is a pigmented area located in the center of the retina that provides the keenest vision. Macular degeneration occurs when the macula starts to break down. It is difficult to study this condition, though, because other animals do not have this structure in the eye. Dr. Johnston hopes that his laboratory can grow the macula of the retina in a dish to allow for further study of this disease.
Another condition that causes blindness is
glaucoma.
Glaucoma is caused by increased pressure in the eye that leads to damage of the
optic nerve.
This damage also results in the loss of neurons in the eye. Kiara observed that ganglion cells in the organoid also die over time. Dr. Johnston thinks there may be a relationship between neuronal death in glaucoma and the observed cell death in their retinal organoids. “When the neurons in the eye lose contact with the brain, they can no longer signal properly, and they die. The same thing might be happening in the dish with our retinal organoids. They try to reach out to the brain, but there is no brain, so they die instead.” If these two mechanisms are similar, Dr. Johnston thinks the retinal organoid could be used to study glaucoma, with the goal of developing better treatments for the disease.
Dr. Robert Johnston is an Assistant Professor at Johns Hopkins University. His laboratory researches light-sensing cells in the eye in both human and animal models. When not in the laboratory, Dr. Johnston enjoys spending time with his family, and occasionally playing video games.
Kiara Eldred is a National Science Foundation graduate research fellow and Howard Hughes Medical Institute Gilliam Fellow for Advanced Study in the laboratory of Dr. Johnston. When not in the laboratory, Kiara enjoys rock climbing and spinning fire (Kiara is trained—don’t try this at home!).
For More Information:
- Eldred, K. et al. 2018. “Thyroid hormone signaling specifies cone subtypes in human retinal organoids.” Science, 362(200):
- Viets, K., K. Eldred., and R. Johnston. 2016. “Mechanisms of Photoreceptor Patterning in Vertebrates and Invertebrates.” Cell, 32(10): 638-659.
- Nakano, T. et al. 2012. “Self-formation of optic cups and storable stratified neural retina from human ESCs.” Cell Stem Cell, 10: 771-785.
- Ng, L., et al. 2001. “A thyroid hormone receptor that is required for the development of green cone photoreceptors.” Nature Genetics, 27: 94-98.
To Learn More:
- Johnston laboratory. https://sites.krieger.jhu.edu/johnstonlab/
- Facts about Glaucoma. National Eye Institute. https://nei.nih.gov/health/glaucoma/glaucoma_facts.
- Facts about Age-Related Macular Degeneration. National Eye Institute. https://nei.nih.gov/health/maculardegen/armd_facts
- American Macular Degeneration Foundation. https://www.macular.org/?gclid=Cj0KCQiA6ozhBRC8ARIsAIh_VC3Hd6L3GLElnXc1pw9gFmQhe-30qksCp66j1oYekEMHsW1FtIa8lAwaAlRUEALw_wcB
- Glaucoma Research Foundation. https://www.glaucoma.org/glaucoma/
- All About Vision. https://www.allaboutvision.com/resources/anatomy.htm
- How Humans See Color. American Academy of Ophthalmology. https://www.aao.org/eye-health/tips-prevention/how-humans-see-in-color
- You and Your Hormones: The Thyroid Gland. http://www.yourhormones.info/glands/thyroid-gland/
- How Your Eye Works. https://lookafteryoureyes.org/eye-care/how-your-eye-works/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

