| |
Nearly four million babies were born in the United States in 2010. These newborns spent an average of nine months growing in the womb before entering the outside world. Across human civilizations, it has been well understood that the
fetus
receives blood and nutrients from its mother during pregnancy. What has been less appreciated, however, is the potential benefit the mother receives from the fetus. This is the starting point for the research conducted by Dr. Kirby Johnson and his colleagues at Tufts University School of Medicine and Tufts Medical Center.
It has been well understood that the fetus receives blood and nutrients from its mother during pregnancy. What has been less appreciated, however, is the potential benefit the mother receives from the fetus.
Every fetus growing inside its mother’s body is fifty percent mother and fifty percent father. We might expect that the mother’s body would reject the fetus as a foreign object. But when the mother is exposed to fetal
antigens
, the mother's body does not mount an immune response. Instead, tolerance occurs and the mother’s body is said to tolerate the fetus growing inside.
Cellular exchange
During the pregnancy, the mother transfers blood and nutrients to the fetus, in part through a process called cellular exchange. But this is not a one-way process. Just as cells are transferred from mother to baby, so are cells transferred from baby to mother. Indeed, research suggests that the bodies of mothers carry for the rest of their lives fetal cells received from their children during pregnancy.
The cells the mother receives from the fetus during pregnancy are different from the mother’s own cells in two important ways: first, they are a generation younger. Second, some of them are stem cells, meaning that they can become a number of different cell types, perhaps influenced by the needs of the mother.
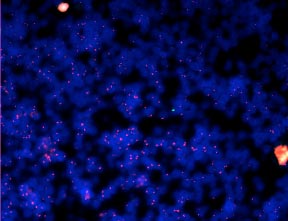
Figure 1. A woman’s adrenal gland containing two X chromosomes stained with red fluorescence. A single male (Y chromosome) from a single fetal cell is stained with green fluorescence and visible in the middle of the image. The blue color is from a stain that makes nuclei visible under the microscope.
The focus of Dr. Johnson and his colleagues’ research is to determine whether the stem cells the mother receives during pregnancy actually function like normal cells in the mother’s body. To do this, they first had to identify the fetal cells, which they did using inheritance from the father, called paternal inheritance.
In humans, this means looking for cells with a Y chromosome (male sex chromosome) in mothers who have given birth to sons. The mother’s own cells will not have a Y chromosome, so any such cells must have been transferred from the fetus to the mother. Other sources of male cells, such as from blood transfusions and organ transplants, must not have occurred, in order to definitively determine that the male cells are from the fetus.
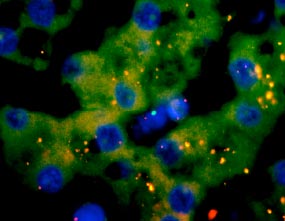
Figure 2. A woman’s liver containing X chromosomes stained with red fluorescence. A single male (Y chromosome) from a single fetal cell is stained with green fluorescence. The blue color is from a stain that makes nuclei visible under the microscope.
In animal models, paternal inheritance can be traced by inserting a gene for a fluorescent protein into the father’s
genome. In the offspring between this father and a normal mother, the cells derived from the father’s genetic material will be clearly visible. Through these initial experiments, Dr. Johnson and his colleagues found that fetal cells are present in nearly every organ of the mother’s body, long after the fetus has grown into an adult.
Through these initial experiments, Dr. Johnson and his colleagues found that fetal cells are present in nearly every organ of the mother’s body, long after the fetus has grown into an adult.
What are these cells doing?
After identifying the locations of fetal cells in the mother’s body, the next step for the researchers was to understand the role of fetal cells in different organs using mouse models. For example, the primary role of skin cells is to produce the protein called
keratin. Once the fetal skin cells have been identified, the researchers can determine whether they are producing keratin like other skin cells. To do this, they use a red
antibody
that binds to the specific protein (in this case keratin). The researchers then look for green skin cells (fetal cells) that are also red (because they are producing keratin). They found that fetal skin cells did in fact appear to function just as the mother’s own skin cells did. Similar experiments were conducted on fetal cells in the thyroid and liver, with comparable results.
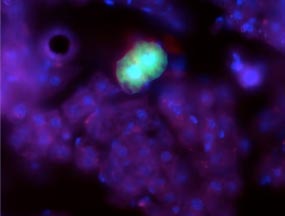
Figure 3. Picture of a green mouse cell in a maternal mouse liver. The mouse cell is stained with green fluorescent protein. The blue color is from a stain that makes nuclei visible under the microscope.
Through this research, Dr. Johnson and his colleagues have shown that fetal cells can be found in virtually all organs of the body with the same apparent structure and function as maternal cells. This is a novel way of looking at pregnancy—not only does the fetus receive blood and nutrients from the mother, but the mother also derives some benefit from the fetus as well.
Dr. Johnson hypothesizes that this maternal benefit may be one reason that women generally live longer than men, a difference found across cultures and civilizations: every time a woman has a child, she receives an influx of young stem cells from the fetus.
Dr. Johnson thinks it is more than just this influx of new cells that can benefit the mother. In some cases, the researchers have found increasing numbers of fetal cells in sick organs compared to healthy organs. So there is not only a passive benefit to pregnancy—an influx of new cells—but an active one—migration of these new cells to damaged or unhealthy tissue. Other researchers have observed blood vessel repair in mice with small skin injuries to determine the role of fetal skin cells. These results so far show that the fetal skin cells are actively involved in tissue repair (Nguyen et al. 2007).
Future?
What does this mean for women who are or may become pregnant? Dr. Johnson wonders whether it might be possible for women to save fetal stem cells for the future in the event that they need them. In this case, rather than receiving foreign cells, it might be possible for the woman to use fetal cells she acquired when she was pregnant. “The possibilities for this technology appear quite open-ended,” remarks Dr. Johnson. “It is an exciting time to be doing this research.”
Dr. Kirby Johnson is an Associate Professor of Pediatrics at Tufts University School of Medicine where he researches cellular exchange between mother and fetus during pregnancy. In addition to his scientific work in the laboratory, Dr. Johnson is also a semi-professional musician. “I do make some money,” Dr. Johnson laughs, “but not enough to quit my day job.” Dr. Johnson is also an avid motorcyclist, “but I always wear a helmet.”
To Learn More:
- Johnson KL, Bianchi DW. Fetal cells in maternal tissue following pregnancy: what are the consequences? Human Reproduction Update 2004;10:497-502.
- Pritchard S, Hoffman AM, Johnson KL, Bianchi DW. Pregnancy-associated progenitor cells: An under-recognized potential source of stem cells in maternal lung. Placenta 2011;32:5298-5303.
- Sanders L. Male DNA found in female brains. Science News. http://www.sciencenews.org/view/generic/id/345443/description/male_DNA_found_in_female_brains
- Krulwich R. Fetal attraction. National Public Radio. http://www.npr.org/blogs/krulwich/2012/05/02/151845273/fetal-attraction
For More Information:
- Women’s health. http://www.womenshealth.gov/pregnancy/
- Fetal health and development. http://www.nlm.nih.gov/medlineplus/fetalhealthanddevelopment.html
- Nguyen Huu S, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci U S A. 2007;104:1871-6.
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Kirby Johnson, PhD
Worldwide birth rates are dropping slightly: the current global crude birth rate is 19.4 births per 1,000 people.

Make sure to thank your mother for all the good things she’s done for you.
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

