|
The largest crystals in the world, found at the Cave of Crystals in Northern Mexico, reach over 36 feet high and weigh nearly 55 tons!
They were discovered accidently in the year 2000 by two brothers mining for lead and silver and have continued to fascinate researchers across a variety of disciplines. What makes crystals so special? It’s the way the charged atoms and molecules, called
ions, bond together in a rigid, orderly pattern.
You’re probably familiar with the most common of crystals, table salt, made of tightly organized sodium and chloride ions and added to almost everything we eat. But did you know that crystals are widely present in the body? Your bones and teeth gain their strength from specialized crystals composed primarily of calcium and phosphorus.

Salt crystal, enlarged many times
http://www.bluesci.org/?p=553
Although the same calcium and phosphorus crystals are found in both bones and teeth, the way they are arranged is very different, and related to their function in the body. Weight-bearing bones like leg bones must be able to withstand the constant pounding of everyday activity; facial bones provide necessary structure but do not bear weight. Teeth, on the other hand, must be able to endure the grinding that occurs every time we eat.
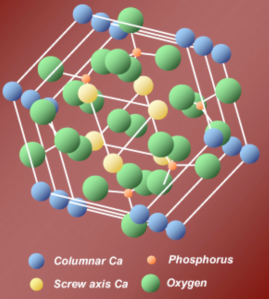
All hard tissues of our skeleton – including tooth enamel – contain crystals of a calcium phosphate salt called hydroxyapatite.
Image courtesy of Dr. Kirkham.
How teeth are built
Teeth are composed of three primary layers:
pulp,
dentin,
enamel.
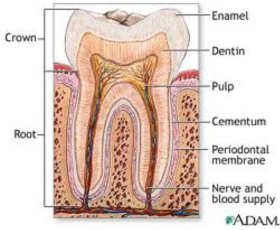
The structure of a tooth
The pulp is the inner soft tissue that contains blood vessels, connective tissue, and nerves. Dentin is a porous, crystallized structure that protects the pulp. It is surrounded by another protective layer, called enamel, whose large crystals make it the hardest and most brittle tissue in the body.
Enamel is produced in association with proteins found in the fluid outside of the enamel-forming cells, known as the
extracellular
(outside the cell)
matrix.
Thanks to their special chemical properties, these proteins are able to self-assemble into larger and larger three-dimensional molecules. As they do so, they attract calcium and phosphate ions that form the crystallized enamel that protects the surface of our teeth.
Almost as soon as our fully-armored teeth emerge in our mouths, though, they are in danger of losing that protection. This is because our mouths are full of bacteria that consume sugar and release acids that slowly dissolve and decay the mineralized
tissue.
This is one reason why it’s so important to brush your teeth at least twice a day, especially if you’ve eaten a lot of sugar. When you go to the dentist for a check-up, the dentist checks for any decay, known as
dental carries
or cavities.
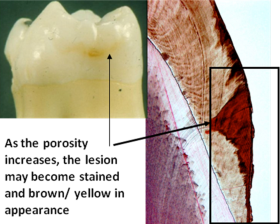
The action of dental plaque in the mouth begins a process that attacks enamel and makes it more porous.
Try as we might to protect our teeth from decay, it is almost inevitable that at some point in our lives we will develop dental caries. Indeed, dental caries is one of the most common disorders, second only to the
common cold. (See the
What A Year! story “
Imagine … A World Without Cavities”, January 2007, here.) Tooth decay is typically treated by drilling out the affected area and removing the decayed tissue. The resulting void is then filled with some other material such as various types of metals, porcelain or synthetic materials. As you may know from your visits to the dentist, this is a minor surgical procedure, but it is not pleasant.
The problem … a solution
If left untreated, dental caries will progress to the soft tissue inside the tooth where it can become incredibly painful and
debilitating.
This is because enamel cannot reproduce its crystals very quickly once they’ve been dissolved by bacteria. But what if our teeth could be stimulated to re-generate the lost crystals? Perhaps we would be able to self-heal our dental caries rather than undergo this procedure to remove the decayed tissue. This is the future imagined by Dr. Jennifer Kirkham and a team of colleagues at the
University of Leeds
in England. These researchers have developed a solution that, when painted onto decaying teeth, regenerates the lost enamel and repairs the decayed tissue to produce healthy teeth.
This latest work is based on years of research on the process of tissue
mineralization.
Dr. Kirkham and her colleagues began studying teeth as a model of biological mineralization because they are readily available for research and the mature enamel contains no cells and is more stable than bone. They investigated inherited genetic disorders, which result in teeth that are not mineralized properly. For example, some cases of
amelogenesis imperfecta,
which results in discolored, fragile, and often painful teeth, are caused by mutations in the self assembling extracellular enamel matrix protein called
amelogenin.
Amelogenin acts as a scaffold around and within which crystals form and teeth are mineralized. Without this structural protein, teeth do not form properly.
Collaboration is key
By studying proteins such as amelogenin and other molecules involved in skeletal tissue mineralization and understanding their properties, Dr. Kirkham and a team of researchers were able to work with colleagues in the Department of Chemistry to develop a set of design principles that would allow them to mimic this biological process in the laboratory.
Before beginning any laboratory experiments, the team of chemists, led by Dr. Amalia Aggeli, used computer modeling to predict which structures would be most likely to mimic the self assembly process that produces scaffolds in bones in teeth, around which crystallization occurs. They designed many different molecules, and set out to create the most promising ones in the laboratory. Once the molecules had been synthesized, they were tested in Dr Kirkham’s laboratories to see how well they could induce the production of crystals.
All tests were conducted using a system developed by Drs. Harvey Goldberg and Graham Hunter and their colleagues in their laboratories in Canada. In this system, the molecule of interest is placed in a chamber with two windows. On one side there is a stream of calcium ions that can enter the chamber through
diffusion;
on the other side there is a stream of phosphate ions that can enter in the same way. The molecule is left in the chamber for seven days before the researchers look to see if any crystals have been created.
Positive and negative controls – actual science at work
The researchers tested the synthesized molecules against a variety of positive and negative controls. Positive controls mimic the response of the system, while negative controls do not produce the desired response. One example of a negative control is agarose gel, which is known not to produce crystals. The researchers tested agarose gel in the same system to make sure they produced the expected result. If, for some reason, the agarose gel did induce crystal formation, the researchers would know something was wrong with their system. Similarly, they used the compound polyglutamic acid as a positive control. Polyglutamic acid is known to produce crystals under these conditions. If it did not, the researchers would know there was a problem with their system. Using these methods, the researchers demonstrated the validity of their system.
For all crystals produced under these conditions, the researchers then did a variety of tests to ensure that the crystal produced exhibited the same properties of the natural crystals found in the enamel of teeth. The researchers then tested each compound for their toxicity to cells. If the compounds were going to be used by humans, they could not have any adverse effects on cells.
P11-4: don’t let the name fool you
After testing many different molecules, the researchers found one, called P11-4, which worked the best. P11-4 has 11 amino acids and 4 negative charges.
The next step was to determine the effects of P11-4 on human teeth that contained areas of decay. The researchers used teeth extracted for orthodontic reasons from human patients and created decay lesions on the teeth that were very similar to naturally-occurring cavities. To do this, they painted nearly the entire tooth with nail varnish, leaving a very small piece of enamel exposed. They then submersed the tooth into an acidic gel. In the acid, the nail varnish protected the tooth except for the area that had been left exposed or unprotected. This area decayed in the same way our natural tissue would decay to produce a cavity.
Throughout the day, the amount of acidity in your mouth fluctuates because the bacteria are active at different times—when you eat, they eat.
Next, the researchers took these teeth with artificial dental caries, placed them into an artificial mouth, and simulated what would happen to the teeth over the course of a day. Throughout the day, the amount of acidity at the tooth surface, measured by
pH,
fluctuates because the bacteria are active at different times—when you eat, they eat. Before placing the teeth in the artificial mouth, the researchers painted some of the lesions with a solution containing the synthesized molecule P11-4. The other teeth served as controls.
The researchers left the artificially-decayed teeth in the artificial mouth with fluctuating pH levels for five days. At the end of the five days, they compared the two groups of teeth to see how well the treatment had worked. Compared to controls, the researchers found impressive signs of repair in the P11-4-treated lesions after only five days. P11-4 works by seeping into the pores of the enamel, where it then forms a gel. Like the proteins found naturally in enamel, P11-4 will only work under particular conditions of pH. The most damaged areas of the tooth are the most acidic and P11-4 is designed to self-assemble and form a scaffold at low pH (below neutral) and in the presence of ions such as calcium.
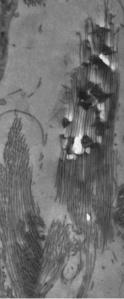
Electron diffraction and elemental analysis indicated presence of hydroxyapatite mineral
This means that P11-4 can be prepared in water, where it is present in its unassembled form as a low viscosity fluid. The fluid penetrates into the micro-pores caused by decay. Inside the pores the conditions of pH and the presence of calcium ions triggers the self-assembly process, forming a 3-dimensional scaffold. The design of the P11-4 molecules means that this scaffold can attract calcium and so promote the formation of crystals inside the decay lesion. Thus, the repair the researchers saw in this experiment was the result of the dental tissue healing from the inside out. Evidence was seen in electron microscope pictures.
Human volunteers in a clinical trial
Finally, it was time to test the effectiveness of P11-4 in humans. To do this, some of Dr. Kirkham’s colleagues at the University of Leeds conducted a small trial with fifteen volunteers who had been diagnosed with dental caries. The clinicians painted the affected areas of the patients’ teeth with a solution containing the synthesized molecule, P11-4. The patients then came back 4, 8, 30, and 180 days later for follow-up. The results so far are very promising. Fourteen of these patients have significant tooth repair as a result of this treatment. The data suggest that P11-4 is repairing the teeth from the inside out. These results confirm the researchers’ original hypothesis.
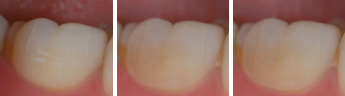
Intra-oral images taken from clinical trial, days 4, 8 and 30 after treatment. The white porous area (decay) disappears, showing lesion repair.
The researchers are currently working with a company to develop P11-4 for human use. The company has recently received approval to market P11-4 in Europe, where it will be available to dentists later this year.
Dr. Jennifer Kirkham is Professor of Oral Biology and Pro-Dean of Research and Innovation for the Faculty Medicine and Health at the University of Leeds in England. She studies the process of biomineralization in human tissue, particularly dental tissue. When not teaching or conducting research, Dr. Kirkham enjoys hiking, sea kayaking, cooking, and embroidery, among other endeavors.
For More Information:
- Kirkham, J. et al. "Self-assembling Peptide Scaffolds Promote Enamel Remineralization." 2007. Journal of Dental Research, 86(5): 426-430.
- Firth, A. et al. "Biomimetic self-assembling peptides as injectable scaffolds for hard tissue engineering." 2006. Nanomedicine, 1(2): 189-199.
To Learn More:
- Centers for Disease Control and Prevention.
http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html
- American Dental Association.
http://www.ada.org/public.aspx
- University of Maryland Medical Center.
http://www.umm.edu/ency/article/001055.htm
- University of California Los Angeles School of Dentistry.
http://www.dent.ucla.edu/pic/members/caries/index.html
Rebecca Kranz with Andrea Gwosdow, PhD www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

