| |
Autism is just one of a range of disorders characterized by deficits in social interaction and verbal and nonverbal communication. Such disorders are commonly known as autism-spectrum disorders, or ASDs, because each individual is affected differently.
Symptoms can range from the milder Asperger’s syndrome to more severe and limiting disorders. There are currently no drug treatments for the social and language aspects of ASDs, largely because their mechanisms of development are poorly understood. These symptoms of the disease are usually managed with therapeutic options such as behavioral therapy, speech therapy and occupational therapy. Medicines may also be used to treat symptoms such as anxiety.
ASDs are widely thought to be caused by a mixture of environmental and genetic factors, although very few environmental factors have been identified or are agreed on. In recent years, much research has focused on finding specific differences in the DNA sequences of patients with ASDs compared to normal populations. Such differences could provide a clue to the genetic basis of the disease. A recent study found that around 1% of ASD patients had a mutation in the SHANK3 gene on human chromosome 22.
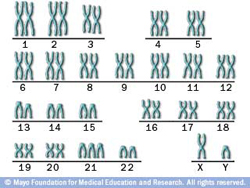
Human chromosome
Reminder: within the nucleus of each of the billions of cells of your body are your chromosomes. There are 23 pairs of chromosomes. Chromosomes are made up of coiled strands of DNA; segments of those strands are called genes. Genes are, among other things, responsible for producing chemicals in your body that guide development and make you “operational” throughout your life. Humans have more than 20,000 genes and scientists have linked gene problems to a number of conditions and diseases.
Lack of Communication?
SHANK3 is known to be involved in the pathways that allow neurons to send messages to each other through electrical impulses. Now, recent experiments conducted by Dr. Joseph Buxbaum and a team of researchers at Mount
Sinai Medical Center in New York showed a clear link between autism-like behaviors and a mutation in the SHANK3 gene.
In order to determine the function of the SHANK3 protein and its possible connection to autism, Dr. Buxbaum wanted to know what would happen if the SHANK3 gene did not exist. To do this, he developed a mouse model known as a “knock-out” mouse because its SHANK3 gene had been deleted, or “knocked-out.” In order to understand the differences between the two groups, Dr. Buxbaum compared the neurophysiology, or the way neurons interact with each other, of the knock-out and control mice.
The researchers observed major neurophysiological differences
between the SHANK3 knock-out mice and the controls. They found that the nerve
cells of the knock-out mice had reduced plasticity compared
to the control mice. Plasticity refers to the ability of nerve cells to change
over time based on one’s experiences by strengthening or weakening the connections
between neurons. Plasticity is measured in millivolts of electricity when one
neuron sends a signal to another (See the video “How neurons communicate”).
When plasticity was measured, Dr. Buxbaum found that the connections between
neurons of the knock-out mice were weaker than those of the normal mice. This
would result in reduced or delayed learning ability that is a common symptom
of ASDs.
Tight Connections
One component of neuron plasticity is the strengthening of the connection between two neurons over time, which is known as long-term potentiation. When a connection between neurons is made stronger, it results in a stronger signal transferred from one neuron to the next. In measurements of electrical impulses, Dr. Buxbaum found that long-term potentiation in the neurons of knock-out mice was weaker and did not last as long in comparison to control mice. This would make it more difficult to learn and retain information.
Once Dr. Buxbaum compared the two groups of mice, the next step was to determine if the brains of the SHANK-3 knock-out mice were different from the brains of the controls. Dr. Buxbaum hypothesized that changes in brain function could cause the observed physiological and behavioral differences. He found that the knock-out mice had a reduced number of neuroreceptors called AMPA receptors (short for, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors). Signals are transmitted between neurons by chemicals known as neurotransmitters. When stimulated by an electrical impulse, the first neuron releases neurotransmitters, which are received by the second neuron through neuroreceptors on its cell membrane. If there are not enough neuroreceptors, as in the case of the SHANK3-deficient mice, the signal received by the second neuron will be weakened.
In addition to comparing the neurophysiology of the two groups of mice, behavioral differences were measured by Dr. Buxbaum’s colleague, Dr. Jacqueline Crawley, who specializes in behavioral neuroscience. Dr. Crawley found that the SHANK3 knock-out mice exhibited social deficits such as reduced interaction between males and females.
Treat the Cause Not the Symptoms
“This is a very exciting discovery,” Dr. Buxbaum remarked, “because it provides a clear molecular explanation for the behavioral changes observed in SHANK3 knock-out mice, and perhaps for some of the behavioral deficits associated with ASDs in humans.” Dr. Buxbaum’s research is now focusing on reversing the behavioral and physiological changes in the SHANK3 knock-out mice using drugs that target AMPA receptor activity. Dr. Buxbaum is conducting experiments to determine if drugs that increase the production of AMPA receptors could reverse the changes observed in SHANK3-deficient mice. If Dr. Buxbaum finds such a drug, the result could ultimate contribute to treatments for ASDs that targets the molecular roots of the disease.
“The goal of this research is to treat not the symptoms of ASDs but their molecular roots,” Dr. Buxbaum explained. Dr. Buxbaum hopes to develop new treatments for autism that target the genetic basis of the disease. Dr. Buxbaum hopes that someday such treatment will be available for patients.
Dr. Joseph Buxbaum is a Professor of Psychiatry, Neuroscience, and Genetics and Genomic Sciences and Director of the Seaver
Autism Center for Research and Treatment at Mount Sinai Medical Center in New York. His research uses genetic and molecular approaches to understand neurological diseases such as autism, schizophrenia and Alzheimer's disease. When not in the laboratory, Dr. Buxbaum enjoys scuba diving, karate, and spending time with his family.
For More Information:
- Bozdagi, O. et al. 2010. “Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication.” Molecular Autism, 1: 15.
To Learn More:
Rebecca Kranz with Andrea Gwosdow, Ph.D. Gwosdow Associates
A note about Wikipedia from Wikipedia: "Wikipedia cannot guarantee the validity of the information found here. The content of any given article may recently have been changed, vandalized or altered by someone whose opinion does not correspond with the state of knowledge in the relevant fields. Note that most other encyclopedias and reference works also have similar disclaimers." http://en.wikipedia.org/wiki/Wikipedia:General_disclaimer.
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. Joseph Buxbaum with Nagahide Takahashi, MD
|

Dr. Joseph Buxbaum
|
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

