|
Human beings have about 40,000 genes.
Hard as it is to believe, sometimes a defect in a single gene
can lead to devastating consequences. This is the case of a rare
childhood genetic
disorder known as Batten
Disease, which currently has no treatment and is inevitably
fatal. But researchers are making breakthroughs in the understanding
of Batten Disease, finding knowledge that may someday lead to
treatments and cures...
Imagine for a moment a disease so brutal
that it can kill a healthy child by the time the child is a teenager.
This is not an infection or something you can catch. It is a
genetic defect known
as Batten Disease.
On
its 46 chromosomes,
the human body has about 40,000 genes that
provide the instructions for making proteins. These
proteins are involved in every single process that goes on in the
body, including, for example, respiration.
We inherit genes from both our mother and father. As humans, we are diploid
organisms, which means that we inherit one copy of each gene from each
parent, so we have two copies of every gene.
But not every gene is perfect; some are defective. And the average
person inherits 3 defective copies of genes out of the total
number of 40,000 genes. This is not usually harmful, because
each person has two copies. If one copy is defective, there is
always the other one.
Batten Disease
Batten Disease is the most common inherited
childhood neurodegenerative disease, though it is relatively
rare – occurring in 2 to 4 out
of every 100,000 births in the US.
A person with Batten Disease will appear
normal until around age five years, when the child will start
to have vision loss. Despite trips to the optometrist for glasses,
eventually the child’s eyesight will fail completely and
the child will go blind. Soon the child will experience cognitive decline; for example, unable to write or read Braille any more.
Then he or she will begin shaking and having epileptic seizures.
Eventually the child’s walking will break down and the
child will have to use a wheelchair or be bedridden. Batten Disease
is fatal in all cases and there are currently no cures and there
are no treatments that will halt the progression of the disease
or slow it down.
You’d
think that a disease as heartbreaking as Batten Disease must
be caused by many defective genes. But Batten Disease comes from
a single defective gene, a gene called CLN3.
Here’s
how it works: A person with only one defective copy of CLN3 gene
will not have the disease, but will be a carrier. If both parents
are carriers and the child unluckily inherits two bad copies
of the CLN3 gene – one from each parent – then
that child does not have a good CLN3 gene, and is positive for
Batten Disease. [For those who studied genetics in Biology class,
Batten Disease is an autosomal
recessive disease, meaning
both parents contribute the defective gene and that defective
gene resides on a non-sex chromosome. With two carrier parents
in this situation, the child has a 1-in-4 chance of getting the
disease.]
Current Research
Dr. David Pearce is perhaps the man
most knowledgeable about Batten Disease in the world. He has
been studying the disease in his laboratory for over ten years.
Dr. Pearce was a researcher in a biochemistry laboratory when
he first read about Batten Disease. “It was time for
me to branch out, and my research at the time lent itself to
the disease,” said Dr. Pearce.
His research was studying the biochemical functions of yeast.
As it turns out, the CLN3 gene has been with us as far back as
yeast on the evolutionary tree. After
preliminary studies on yeast, Dr. Pearce moved on to mice. “Unfortunately
with a juvenile neurological disorder such as Batten Disease,” he
said, “we have to use a mammalian model.”
Dr.
Pearce used a mouse model that did not have the CLN3 gene, just
like the real Batten Disease children. These mice are called knockout mice
because one of the genes has been “knocked out.” These
mice develop some of the same symptoms as the children with Batten
Disease. Dr. Pearce and his researchers study the brains of these
mice in order to figure out what happens with the disease as
it progresses.
One way
to test cognitive abilities in mice is to use the log-rolling
test. This test uses a metal rod that rolls in the water. The
mice walk on the rod and it will spin faster and faster until
eventually the mice fall off. Mice with Batten Disease will fall
off much sooner than normal mice because of their decreased cognitive
function.
Through
their studies, Dr. Pearce and his lab found the brain cells of
the mice with Batten disease are overexcited. What does this
mean? To understand it, let’s
understand how brain cells communicate with each other. [Note: visitors to
the What A Year! website can get more information
on how brain cells communicate in several of our stories: Autism 12/07, Multiple
Sclerosis 01/08, Epilepsy 02/07,
and Parkinson’s
Disease 11/06.]
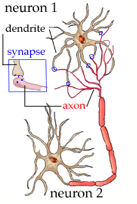
To communicate with
each other, brain cells send electric impulses from one neuron
(brain cell) to the other. One neuron will get excited, fire
a signal to the next neuron, and then inhibit (stop) itself
until it gets another signal from a neighboring neuron. But
if a neuron gets excited and remains excited, continually firing
signals, the cells will eventually burn out and die, because
neurons need to be able to switch off and have some down time
from constant activation. Dr. Pearce suspects that a loss of
brain cells may play a role in the degeneration of children with
Batten Disease.
When a neuron
fires a signal, it actually sends chemicals called neurotransmitters over
the gap, or synapse,
that separates the brain cells from one another. Dr. Pearce found
that neurons in Batten Disease mice are over-sensitive to one
particular neurotransmitter, called glutamate. Dr.
Pearce and his team were able to stop Batten Disease degeneration
in mice by inhibiting a specific glutamate receptor on
the cell membranes of brain cells. They are still doing extensive
research on mice, but hope to soon extend their research to clinical
trials in humans.
Dr. Pearce
and his researchers have also found an autoimmune component
to Batten Disease. They found that mice with Batten disease
make antibodies to their own proteins. Dr. Pearce hypothesized
that these autoantibodies or self-antibodies lead to the body’s
no longer recognizing itself, allowing an autoimmune response
to occur. Dr. Pearce demonstrated the presence of these autoantibodies
in children with Batten Disease.
To study the autoimmune aspects
of Batten Disease, Dr. Pearce developed immunosuppressed mice.
These mice did not have the ability to make these autoantibodies.
Interestingly, the progression of Batten Disease was slowed in
immunosuppressed mice. Dr. Pearce is now also studying immunosuppression
in children with Batten Disease.
Dr. Pearce explains, “Batten
Disease deals with many parts of the brain, and unfortunately
I don’t envision this problem
being solved by just one drug.” Dr. Pearce hopes drugs
will eventually be available to slow the progression of the disease.
For now, there are support groups around the world for the families
of children with Batten Disease.
Dr. David
Pearce is an Associate Professor of Biochemistry and Biophysics
in the Center for Neural Development and Disease at the University
of Rochester in New York and the scientific advisor for
the Batten
Disease Support and Research Association. Dr. Pearce
completed his undergraduate and doctorate work in England
before coming to the United States as a post-doctoral fellow.
He chose science as a career because it was the easiest subject
for him in school and also the most interesting. |

