| |
Highlights
- In order to hear, our brain converts sound waves into electrical signals by changing the gradient of positive and negative charges in the cell.
- Researchers identified the major protein involved in the translation of vibration into electrical signals as TMC1 by comparing electrical signals in the hair cells of control mice with those of mice with mutations in the TMC1 gene.
- The researchers were able to predict the structure of the TMC1 protein, which suggested that TMC1 forms a pore that allows positive ions into the hair cell, stimulating the process that transforms vibrations into electrical signals.
How does the brain hear? Or to be more scientific, how does the brain convert sound waves into electrical signals that the brain can understand? Dr. David Corey, Professor of
Neurobiology
at Harvard Medical School and Dr. Jeffrey Holt, Professor of
Otolaryngology
and
Neurology
at Harvard Medical School, have spent their careers devoted to these questions. Recently, Drs. Corey and Holt and a team of colleagues in their two laboratories including Drs. Bifeng Pan, Nurunisa Akyuz, and Xiao-Ping Liu made a major breakthrough in the scientific understanding of our sense of hearing. They identified the major protein involved in the translation of vibration into electrical signals, opening up new possibilities for understanding how this protein and many other proteins in the same complex work together to allow us to hear. Moreover, this further understanding of hearing paves the way for the development of new treatments for hearing loss.
How We Hear
In order to understand the research of Drs. Corey and Holt, let’s begin with some background information on human hearing. The part of the ear that we see on our faces is only the beginning of the structures that allow us to hear. The ear actually has three major parts: the
outer ear,
the
middle ear,
and the
inner ear.
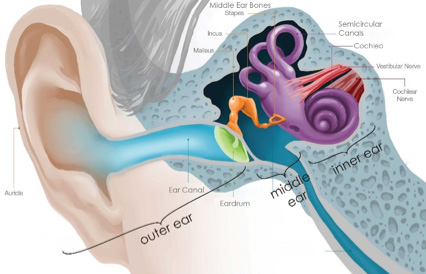
Figure 1. Structures of the ear.
[Adapted from:https://www.earq.com/hearing-loss/ear-anatomy#all
Our ears and the canal they connect to are collectively known as the outer ear. The
ear canal
ends at the
eardrum,
which connects to three small bones within the middle ear. These middle ear bones carry the vibration of sound from the eardrum to the inner ear, where the
cochlea,
named for its snail-like structure, converts the sound to electrical signals. The cochlea connects to the eighth
cranial nerve,
known as the
vestibulocochlear nerve,
which carries these electric signals to the brain.
How the vibrations are converted into electrical signals within the cochlea has not been fully understood. The primary mechanism by which this happens was known to be through
hair cells,
specialized cells so-named because they carry bundles of
stereocilia
that look like little tufts of hair. “We know that vibrations in the inner ear fluid move these hair-like projections called stereocilia,” explains Dr. Corey. “But once the stereocilia are moved, we don’t know exactly how the sound vibrations are converted into electrical signals that go to the brain.”
Experimenting with Genes
The researchers did have some idea of how this process might work, because they already knew the basics of how electric signaling works in the
nervous system.
In the nervous system, electrical signals are transmitted by changing a gradient of positive and negative charges. When an individual neuron is made more positive by an influx of positive ions like sodium or calcium, this changes the electrical landscape of the cell, resulting in a signal that travels from one end of the cell to the other.
Based on their understanding of the nervous system, Drs. Corey and Holt knew that a similar process was at work in hair cells, and they hypothesized that there was a specific protein involved in the transfer of ions into the hair cells.
Drs. Corey and Holt had a clue about one protein in particular, because in 2002 two separate research teams identified distinct
mutations
in a specific gene that led to hereditary deafness. This gene coded for a protein known as trans-membrane channel-like protein 1 or TMC1.
The name of the TMC1 protein does a good job at describing its function, which is to act as a channel through the membrane of the hair cell. Specifically, the researchers hypothesized that TMC1 would form a pore that allows positive ions into the hair cell, stimulating the process that would transform vibrations into electrical signals.
In order to better study hair cells, Drs. Corey and Holt used
mouse models
of hearing and hearing impairment. This is because the basic mechanisms of hearing are the same in all vertebrates, and the process of hearing in mice is very similar to that of humans, especially at the level of the hair cell.
To begin, the researchers isolated just the hair cells from normal mice and looked at them under the microscope. They put an
electrode
on the cell to measure electrical activity and wiggled the bundles of cilia back and forth to create a stimulus similar to sound. In this way, the researchers could record the electrical signals generated by the hair cell in response to vibration. The researchers tested this approach first in normal mice, and then in mice which had been genetically engineered to have mutations in the TMC1 gene.
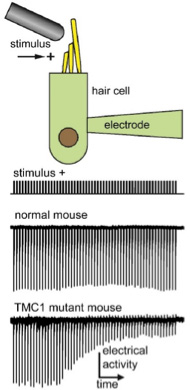
Figure 2. Electrical recordings from normal and mutant mouse hair cells. [Source: Dr. Jeffrey Holt]
Each TMC1 gene encodes 760
amino acids
that are strung together in a certain order to create the TMC1 protein. Drs. Corey and Holt wanted to test different mutations in the gene, but they couldn’t test all the possibilities. They had to guess based on their understanding of similar proteins.
Fortunately, a similar ion channel had just been described at about the same time they were doing these experiments. The researchers were able to use that information to make their best guesses about which parts of the gene would be most involved with the flow of ions in and out of the cell. In addition, Dr. Holt and his laboratory had already identified several mutations in the TMC1 gene that were known to cause deafness, so the researchers decided to test those mutations as well.
In total, Drs. Corey and Holt chose 17 different mutations of the TMC1 gene that they wanted to test. To do this, they had to develop 17 different variations of the TMC1 gene and inject them, one-by-one, into the ears of mice that lacked functional TMC1 protein. By comparing the electrical signals in the hair cells of normal mice with those of mice injected with different mutations, the researchers could determine how a given mutation affected electrical signaling.
A Protein is Born
These carefully constructed experiments lasted five years. By the end, Drs. Corey and Holt came to a breakthrough in the field of hearing and hearing loss. They were able to predict the structure of the TMC1 protein, which suggested that a complex of two TMC1 proteins assemble together with 6 to 8 other proteins. The two TMC1 proteins are arranged back-to-back, in this model, and each has a large pore.
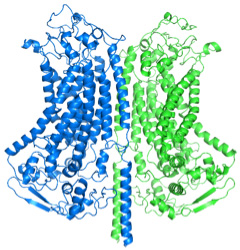
Figure 3. Proposed structure of the TMC1 dimer, with two TMC1 proteins back-to-back.[Source: Dr. David Corey]
“This structure makes sense given everything else we know about hair cells,” explained Dr. Holt. For example, there are some
antibiotics
that can affect hair cells without affecting any other types of cells in the body. That’s because the pore in the hair cell is so much bigger than in other cells, which allows the antibiotic to actually enter the hair cell and cause damage. In other cells, transmembrane pores wouldn’t allow such a large molecule to enter.
The researchers imagine hearing working this way: when the stereocilia move in response to vibrations of sound, this causes little strings attached to the TMC1 protein to pull on the complex, opening up the pores thousands of times a second. When the pore opens up, this allows greater flow of positive ions into the hair cell, which results in an electrical signal that is sent to the brain.
“We have been working on this problem for over 40 years,” comments Dr. Corey. “And now we can begin to identify the function of all these other proteins of the complex.” Indeed, Dr. Corey likes to think of our current knowledge of the vertebrate hearing system as a bunch of watch parts. “With this study, we have gathered all the watch parts. Now we have to figure out how each one functions so we can begin to put the watch together.”
The researchers also hope these findings will lead to new avenues of research into hearing loss, and potential treatments.
Dr. David Corey is Professor of Neurobiology at Harvard Medical School. His research focuses on channel proteins found in the hair cells of the inner ear, and how these proteins relate to hearing and hearing loss. When not in the laboratory, Dr. Corey enjoys sailing.
Dr. Jeffrey Holt is Professor of Otolaryngology and Neurology at Boston Children’s Hospital and Harvard Medical School. Dr. Holt worked in Dr. Corey’s laboratory during his post-doctoral fellowship after receiving his Ph.D. Both Dr. Corey and Dr. Holt shared a fascination with hearing and hair cells, and they have continued to collaborate on their research ever since.
For More Information:
- Corey, D., N. Akyuz, and J. Holt. 2018 “Function and dysfunction of TMC channels in inner ear hair cells.” Cold Spring Harbor Perspectives in Medicine. doi: 10.1101/cshperspect.a033506
- Pan, B. et al. 2018. “TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells.” Neuron, 99: 736-753.
- Pesheva E. “Ending 40-year quest, scientists reveal ‘hearing’ protein.” Harvard Gazette August 22, 2018. https://news.harvard.edu/gazette/story/2018/08/hearing-protein/
- Grens K. 2018 “The biggest science news of 2018: Hearing channel protein finally identified.” The Scientist https://www.the-scientist.com/news-opinion/the-biggest-science-news-of-2018-65256
To Learn More:
- Corey Laboratory. http://corey.med.harvard.edu/index.html
- Holt/Géléoc Laboratory. https://www.holtgeleoclab.com/
- National Institute on Deafness and other Communication Disorders. https://www.nidcd.nih.gov/health/hearing-ear-infections-deafness
- “Hair Cell Regeneration and Hearing Loss.” https://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=94
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Left to Right: Drs. Jeff Holt, David Corey, Gwen Geleoc

Dr. Bifeng Pan

Dr. Nurunisa Akyuz

Dr. Xiao-Ping Liu
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

