| |
It’s true that we all need a little space sometimes. Well, it turns out that our bones do, too.
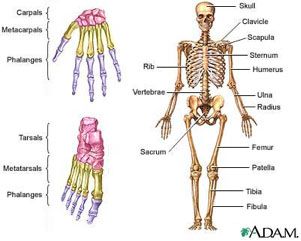 Figure 1 Human skeleton, courtesy of the National Library of Medicine
There are 206 named bones in your body: they support you and allow for movement. Any place where two bones come together is known as a joint , or articulation. If the two bones were simply attached to each other, with nothing soft in between, the grinding of the two bones against each other as a result of movement would be extremely painful. Instead, all bones need a little space separating them from other bones. The space between bones is filled with a layer of connective tissue called cartilage. Cartilage is made of cells called chondrocytes. A special brand of chondrocytes called articular chondrocytes is found only in the cartilage at the ends of your bones. They secrete a fluid known as synovial fluid that fills the spaces between bones. So in a healthy body, every two bones are separated by a little space filled with synovial fluid. But if something goes wrong with the articular chondrocytes in a joint, there will be a problem there. Figure 1 Human skeleton, courtesy of the National Library of Medicine
There are 206 named bones in your body: they support you and allow for movement. Any place where two bones come together is known as a joint , or articulation. If the two bones were simply attached to each other, with nothing soft in between, the grinding of the two bones against each other as a result of movement would be extremely painful. Instead, all bones need a little space separating them from other bones. The space between bones is filled with a layer of connective tissue called cartilage. Cartilage is made of cells called chondrocytes. A special brand of chondrocytes called articular chondrocytes is found only in the cartilage at the ends of your bones. They secrete a fluid known as synovial fluid that fills the spaces between bones. So in a healthy body, every two bones are separated by a little space filled with synovial fluid. But if something goes wrong with the articular chondrocytes in a joint, there will be a problem there.
Eroding Cartilage
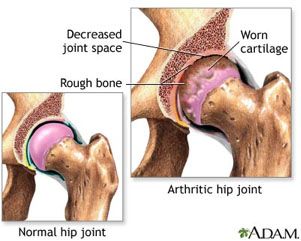 Figure 2 Normal vs Arthritic Hip Joint, courtesy national Library of MedicineWith the constant beating that certain joints, like the hip or knee, receive over a lifetime, the cartilage that separates the bones begins to wear away. As cartilage degenerates, the synovial fluid filling the space between the bones begins to disappear, and the bones move closer together. The proximity of bone to bone results in the painful condition we know as arthritis. Arthritis is actually a term used to describe any inflammation or pain in the joints. There are many types of arthritis that have different causes, many of which we do not yet understand. The most common type of arthritis, affecting over 20 million Americans, is osteoarthritis. If you know someone with arthritis, they will tell you that it can be a very painful condition. Figure 2 Normal vs Arthritic Hip Joint, courtesy national Library of MedicineWith the constant beating that certain joints, like the hip or knee, receive over a lifetime, the cartilage that separates the bones begins to wear away. As cartilage degenerates, the synovial fluid filling the space between the bones begins to disappear, and the bones move closer together. The proximity of bone to bone results in the painful condition we know as arthritis. Arthritis is actually a term used to describe any inflammation or pain in the joints. There are many types of arthritis that have different causes, many of which we do not yet understand. The most common type of arthritis, affecting over 20 million Americans, is osteoarthritis. If you know someone with arthritis, they will tell you that it can be a very painful condition.
Osteoarthritis is caused by the degeneration of the joints because of the malfunctioning of articular chondrocytes. In osteoarthritic patients the articular chondrocytes no longer secrete synovial fluid, and the bones come almost in contact with each other. Osteoarthritis is characterized by mild to severe pain in the joints due to the increasing proximity and rubbing of the bones. In mild cases, there are several drugs available to decrease pain levels. In the most severe cases, the only real solution is total joint replacement surgery. There is no treatment on the market for intermediate pain levels, and all of the treatments currently available focus on reducing the symptoms of osteoarthritis instead of addressing the root cause. This is because the exact mechanism or reason for the malfunctioning of articular chondrocytes that causes osteoarthritis is poorly understood. There are most likely environmental and genetic factors that contribute to osteoarthritis. A lot of research is going on in this area.
To test the possible contribution of genetics to the development of osteoarthritis, researchers (Min et al, 2005) from England and the Netherlands conducted a ten-year genetic study of patients with osteoarthritis. The study, published in 2005, found that a genetic mutation in one specific gene, the Frzb gene (yes, it’s pronounced the way you think it is), increased the likelihood of developing osteoarthritis. Frzb encodes for proteins that block the action of another protein called beta-catenin. Beta-catenin is found in articular chondrocytes and other bone cells. Without the Frzb gene, mice were more likely to develop osteoarthritis. This 2005 study demonstrated a possible link between beta-catenin and the development of osteoarthritis, but it was still unclear exactly what role beta-catenin played.
Understanding Beta-catenin
This is where Dr. Di Chen of the University of Rochester Medical School comes in. Dr. Chen wanted to determine the role of beta-catenin in the development of osteoarthritis and to develop a mouse model of the disease. Other scientists had made "knock-out" mice, whose beta-catenin gene had been deleted from the genome. If there were no beta-catenin gene, there would also be no beta-catenin in the body. These mice died as embryos, indicating the importance of the beta-catenin gene to bone growth and development.
As a next step, Dr. Chen developed a transgenic mouse model that over-expressed (= produced too much of) the beta-catenin gene. The transgenic method is a technique of inserting foreign genetic material into the genome of an organism. The transgenic mice that Dr. Chen developed had genes that contain genetic material foreign to the normal mouse gene. The foreign genetic material had a section that acted as a switch for the beta-catenin gene and could be turned on when the animals were treated with a drug called tamoxifen. When treated with tamoxifen, the transgenic mice were induced to over-express beta-catenin.
Arthritis in Mice
Dr. Chen conducted experiments with these mice, taking advantage of the genetic switch. He treated one group of mice with tamoxifen, inducing the over-expression of beta-catenin and starting the disease process. He compared the development of the treated group to the control group of non-transgenic mice that had not been induced with the drug and had normal levels of beta-catenin. After two months, Dr. Chen noticed an increase in beta-catenin levels in the articular chondrocytes in the treated mice. In 5-month-old treated mice, he observed mild degeneration of articular cartilage in their knees. Eight-month-old tamoxifen-treated mice had severe cartilage damage in their knees. These changes to the joints of mice with high levels of beta-catenin were identical to what is observed in people with osteoarthritis. In comparison, the control mice had no damage to the cartilage or knee joints at any point in time. Dr. Chen had succeeded in creating a mouse model of osteoarthritis. Dr. Chen’s experiments also clearly indicated that beta-catenin plays a significant role in the development of osteoarthritis.
Human Knees
The next step for Dr. Chen and his colleagues was to take samples of joint tissue from human patients and examine their levels of beta-catenin. They took samples from patients suffering from osteoarthritis who had undergone knee surgery as the treatment group and samples from amputee patients as the control group. Dr. Chen found that the patients with osteoarthritis did indeed have heightened levels of beta-catenin in their articular chondrocytes while the control patients did not. These results provided further evidence that an increase in beta-catenin levels in articular chondrocytes adversely affects the joints and causes osteoarthritis.
It is important to remember that the development of osteoarthritis is not simply genetic. It is a known phenomenon that people with meniscus injuries, an injury resulting from a tear in articular cartilage, almost always develop osteoarthritis later in life. In looking at cartilage tissues, Dr. Chen found that patients recovering from meniscus injuries had increased beta-catenin activity in their articular chondrocytes. While the patients recovered from the injury, Dr. Chen believes that the increase in beta-catenin may put them at a higher risk for developing osteoarthritis later on in life. One of Dr. Chen’s current goals is to develop a drug to block the increase of beta-catenin after injury. In this way, young patients recovering from an injury will not be at risk for osteoarthritis later on in life due to increased beta-catenin activity in response to the injury.
Another important factor that can cause osteoarthritis is obesity. The joints of obese people must support much more weight and work much harder than non-obese people. The condition where joints support too much weight is known as mechanical loading. In another set of ongoing experiments, Dr. Chen is testing mice with mechanical loading to see if they develop increased levels of beta-catenin. Dr. Chen expects these mice will secrete large levels of beta-catenin and will develop osteoarthritis, just like people.
Dr. Di Chen is a Professor of Orthopaedics at the University of Rochester Center for Musculoskeletal Research. He received his medical degree in China before coming to the United States to get his Ph.D. Dr. Chen conducted his doctoral work on bone biology at the University of Louisville. He completed his post-doc at the University of Texas where he studied bone formation. He then moved to the University of Rochester in 2003 to study chondrocyte bone biology, bone fracture and fracture-healing. With the success of his transgenic mouse model, Dr. Chen is now looking more closely at the development of osteoarthritis. Dr. Chen became interested in science and medicine at an early age in China. “My father was a doctor, my mother was a nurse, and I was interested in science,” Dr. Chen commented. In his free time Dr. Chen likes to travel with his wife and kids.
To Learn More:
For More Information:
- Zhu M., et al. “Activation of ?-Catenin Signaling in Articular chondrocytes Leads to Osteoarthritis-like Phenotype in Adult ?-Catenin Conditional Activation Mice. Journal of Bone and Mineral Research (2008).
- Min, JL, et al. “Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites.” Arthritis & Rheumatism. 52(2005): 1077-1080.
Written by Rebecca Kranz with Andrea
Gwosdow, Ph.D. Gwosdow
Associates
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. Di Chen of the University of Rochester Medical Center

Dr. Mei Zhu of Tianjin Medical University presents results in a scientific poster session
 What Is Osteoarthritis? What Is Osteoarthritis?
Video courtesy of About.com
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

