Last month, What A Year! explored the work of Dr. Korkut Uygun from the Center for Engineering in Medicine at Massachusetts General Hospital and Harvard Medical School, whose team successfully transplanted rat livers after storing them for 72 hours—an 800 percent increase in time over current
organ transplant
procedures. The researchers avoided tissue damage by keeping the livers unfrozen at freezing temperatures, or
supercooled.
This month, we highlight the work of Dr. John Bischof, who trained in that laboratory with Dr. Mehmet Toner. Dr. Bischof’s research uses
iron oxide
nanoparticles
to address the challenges of
organ
damage during the re-heating process.
Every organ is distinct in its form and function—what types of cells it contains, what functions they perform in the organ, and how much fluid they contain, to name a few. For this reason, organ preservation techniques must be adapted and modified to address the specific challenges posed by each organ. For example, the liver is a relatively large organ with many different types of cells and limited fluids. Dr. Uygun and his team developed a method to avoid organ damage through supercooling, which was successful in livers. This same technique may not work in other organs that have different cell and tissue structures, especially if the time of storage needs to be increased from hours to months or potentially years. This is why collaborations between researchers to develop multiple approaches to similar problems is so important and warrants a different approach, such as the one developed by Dr. Bischof.
Dr. Bischof, Distinguished McKnight University Professor, Carl and Janet Kuhrmeyer Chair in Mechanical Engineering, and Interim Director of the Institute for Engineering in Medicine at the University of Minnesota, is a self-described “heat transfer guy.” He studies how live tissues change
phase,
and specifically how heat can help preserve or destroy tissues. His recent research has focused on the use of iron oxide nanoparticles, literally 10 to 20 nanometers in diameter, that can be used to uniformly heat tissues that have been preserved in a glassy solid state, a process known as
vitrification.
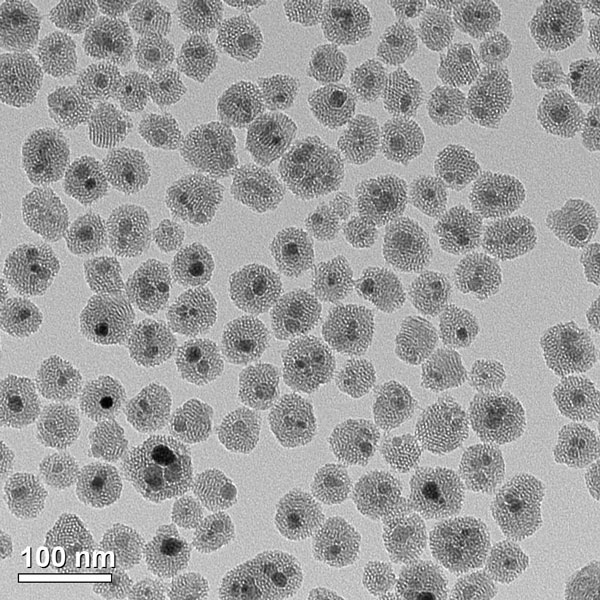
Figure 1. Iron oxide nanoparticles used in nanowarming that are made biocompatible due to coating with silica (Coating work performed in collaboration with Dr. Christy Haynes)
Photo credit: Patrick O’Leary, University of Minnesota
Vitrification presents many problems. However, much progress has been made in how live tissues can be preserved in this solid, glass-like state. Researchers have identified specific chemicals, known as
cryoprotectants,
that prevent cold damage during the cooling process. But cooling is only the first part of the process. In order for vitrification to lead to an increased supply of organs for donation, the organs must also be brought back to room temperature without incurring tissue damage.
When an ice cube is taken out of the freezer and placed in a glass of water it almost always cracks. This cracking is due to thermal stress from temperature differences between the rather warm surface where the melt is occurring vs. the center of the ice cube where it is still cold from storage in the freezer. By dispersing iron oxide nanoparticles throughout an ice cube we can melt the whole ice cube at the same time without cracking. The same idea is now being applied to large tissues and organs with our new nanowarming approach.
This is the focus of Dr. Bischof’s work. Dr. Bischof and his team utilized basic principles of physics and chemistry to develop a method for using nanoparticles to create heat inside tissues. In this case, the iron oxide nanoparticles, when placed within an alternating
magnetic field
(i.e. a radiofrequency field), will either physically rotate, or flip dipoles, to create heat. This is why the researchers chose to fill the biocompatible polymer-coated nanoparticles with iron oxide. The researchers hoped that the combination of iron oxide particles spread within frozen tissue and the application of
electromagnetic waves
would cause the nanoparticles to uniformly heat the surrounding tissues.
To test their hypothesis, Dr. Bischof and his team used blood vessels found in the circulatory system and the heart. These vessels were frozen and then reheated using iron oxide nanoparticles and cryoprotectant solution. In a series of experiments, the researchers tested different types of cryoprotectant solutions, different concentrations of iron oxide nanoparticles, and different rates of heating by radiofrequency exposure. They measured the viability and function of the tissue after each experiment by measuring the
enzymes
produced and the elasticity of the blood vessels as compared to healthy blood vessel controls.

Figure 2. Experimental set up with heart valve in one vial and iron oxide solution in the other
Photo credit: Patrick O’Leary, University of Minnesota
One major challenge for the researchers was to avoid what Dr. Bischof calls the “ice cube effect.” When an ice cube is taken out of the freezer and placed in a glass of water it almost always cracks. This cracking is due to thermal stress from temperature differences between the rather warm surface where the melt is occurring vs. the center of the ice cube where it is still cold from storage in the freezer. By dispersing iron oxide nanoparticles throughout an ice cube we can melt the whole ice cube at the same time without cracking. The same idea is now being applied to large tissues and organs with our new nanowarming approach.
Another challenge for the researchers was the distribution of the nanoparticles. In some applications of nanoparticles, such as cancer treatment, the goal is for the particles to infiltrate the tissues of the entire organ. This is not what Dr. Bischof and his team wanted. Instead, the team needed to find a way for the nanoparticles to be distributed evenly throughout the inside of the blood vessels but not into the cells. This is because the researchers want to wash all the nanoparticles out once the process is complete, so that only a functional blood vessel is left.
Dr. Bischof and his team were able to meet these two important challenges and successfully warmed blood vessels that maintained their viability and function. They were able to reach a maximum and uniform rate of 100 degrees Celsius per minute in vials up to 50 mL in size, a rate ten times more than what has previously been achieved by gold standard convection in the center of a similar vial. And the nanoparticles were removed at the end of the process, leaving a functional blood vessel with little indication of the process it had just undergone.
Now that Dr. Bischof and his team have demonstrated the effectiveness of iron oxide nanoparticles in warming frozen tissues, the next steps involve collaborations with researchers who have developed methods for freezing organs. In particular, Dr. Bischof is collaborating with Dr. Greg Fahy, who has successfully vitrified rabbit kidneys but has not been able to restore complete function after achieving vitrification. Together, Dr. Bischof hopes that they, with surgical colleagues such as Dr. Erik Finger, will successfully create a model for organ transplantation that could be used in humans. If the kidney model is successful, Dr. Bischof plans to initiate similar collaborations with colleagues working on other organ systems.
Dr. Bischof is the Distinguished McKnight University Professor, Carl and Janet Kuhrmeyer Chair in Mechanical Engineering, and the Interim Director of the Institute for Engineering in Medicine at the University of Minnesota. Dr. Bischof leads the Bioheat and
Mass
Transfer Laboratory, where his research interests include
nanotechnology,
biopreservation, and the thermal and mechanical properties of live tissues. When not in the laboratory, Dr. Bischof enjoys spending time with his family.
For More Information:
- Manuchehrabadi, N., et al. 2017. “Improved Tissue Cryopreservation Using Inductive Heating of Magnetic Nanoparticles” Science Translational Medicine, 9(379), doi: 10.1126/scitranslmed.aah4586
- Etheridge, M.L., et al. 2014. “RF Heating of Magnetic Nanoparticles Improves the Thawing of Cryopreserved Biomaterials.” Technology, 2:229-242.
- US Scientists Find Way to Safely Thaw Cryopreserved Tissues. http://www.reuters.com/article/us-health-transplants-organs-idUSKBN1685E2
- Groundbreaking Technology Successfully Rewarms Large Scale Tissues Preserved at Low Temperatures https://cse.umn.edu/news-release/groundbreaking-technology-successfully-rewarms-large-scale-tissues-preserved-low-temperatures/
To Learn More:
Websites
- Dr. Bischof’s web page.
http://www.me.umn.edu/people/bischof.shtml
- Bioheat and Mass Transfer Laboratory.
https://sites.google.com/a/umn.edu/bhmt/home
- Organ Preservation Alliance.
https://www.organpreservationalliance.org
- American Society of Transplantation.
https://www.myast.org/about-ast/about-ast
Publications
- Lewis, J.K., et al. 2016. “The Grand Challenges of Organ Banking: Proceedings from the First Global Summit on Complex Tissue Cryopreservation.” Cryobiology, 72:169-182.
- Berendsen, T.A., et al. 2014. “Supercooling Enables Long-Term Transplantation Survival Following 4 Days of Liver Preservation.” Nature Medicine, 20:790-794.
- Song, Y.C., et al. 2000. “Vitreous Cryopreservation Maintains the Function of Vascular Grafts.” Nature Biotechnology, 18:296-299.
- Fahy, G.M., et al. 1984. “Vitrification as an Approach to Cryopreservation.” Cryobiology, 21:407-426.
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com

